
Titration of Antibodies – Flow Cytometry Staining Protocol
Methods and Principles from our Scientific Staff
Background
Titration is the process of identifying the correct concentration of antibody to use for a given assay. This ensures the antibody performs within acceptable parameters including reducing negative effects and waste. Titration for flow cytometry is used to determine the antibody amount and concentration resulting in the highest signal of the positive population along with the lowest signal of the negative population. It is also the best way to eliminate nonspecific binding of an antibody.
Why Do It?
The precise amount of an antibody is essential to proper titration.
Too much antibody will result in an increase in the background non-specific (or low affinity) binding, reducing the sensitivity of the fluorescent measurement.
Too little antibody will result in a decrease in the positive signal, again reducing the sensitivity of the fluorescent measurement.
Figure 1. (Left) As concentration of antibody increases there is greater separation of positive and negative cells based on fluorescence intensity.
Leinco Product C119, Anti-Human CD8 (Clone: UCHT-4) – FITC
How To Do It
Titration experiments are easy to perform, and there are several papers that discuss this process1,2. This protocol has been optimized for 1×106 cells in 50 µl final volume. All staining is performed on ice, in the dark, for 20 minutes. Identify the 1x concentration, which is the manufacturer’s recommended concentration for using their antibody in a given assay. These concentrations can either be a volume (10 µl per 100 µl) or a concentration (0.1µg/µl). Based on the starting concentration, generate a serial dilution series of 4 to 8 points. It is often good to include a 2x concentration to ensure the antibody is working well. If the optimal concentration is 2X the recommended usage, this can suggest a problem.
Solutions Needed
1. Staining Buffer: Phosphate Buffered Saline with 0.5% Bovine Serum Albumin (Fraction V) (See Leinco Part Number: F1175)
2. Fixation Buffer: 1-5% formaldehyde in PBS (See Leinco Part Number: F1194)
3. Blocking Buffer: 5-10% Normal (species) Serum in Staining Buffer. The species to use is based on the origins of the antibody being used. (Leinco has many Normal Serum species available).
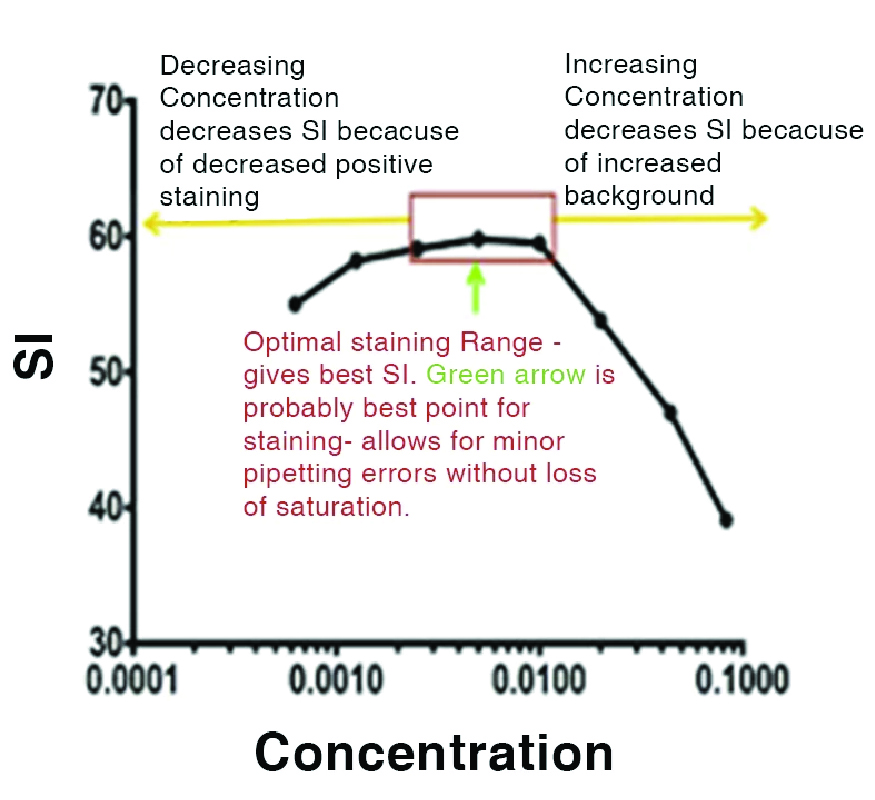
Typical Results
As is shown in this curve, the titration reveals the optimal concentration (shown in green). As the antibody is increased, the staining index decreases because of the increase in background binding. On the other side, as the concentration decreases, the staining index decreases because the antibody is not at saturating concentrations.
References
1. Hulspas R, O’Gorman MR, Wood BL, Gratama JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry B Clin Cytom 2009;76:355-64.
2. Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A 2006;69:1037-42.
3. Telford WG, Babin SA, Khorev SV, Rowe SH. Green fiber lasers: an alternative to traditional DPSS green lasers for flow cytometry. Cytometry A 2009;75:1031-9.
Special Acknowledgment and Thanks to the Siteman Cancer Flow Cytometry Core Facility Core Director Mr. Bill Eades and The University of Rochester Medical Center Flow Cytometry Shared Resource Share Resource Direct Dr. Timothy Bushnell, Ph D for their review and input on this technical document.

