Monoclonal Antibody Manufacturing
Scalable Antibody Production From Hybridomas
Antibody Production Services
Leinco Technologies offers scalable monoclonal antibody production solutions. From small-scale hybridoma development to large-scale antibody manufacturing for IVD raw materials, we adapt to your project’s specific needs. Experience superior quality and purity with our ISO-certified processes while maximizing efficiency and controlling costs.
Key Benefits
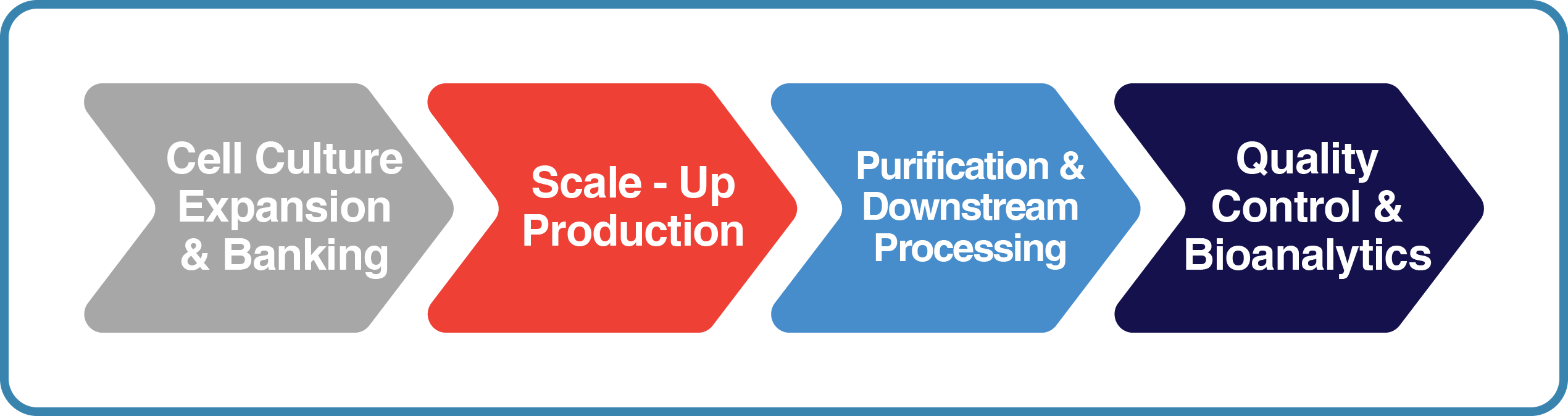
How it Works
What to Expect
Every project starts with a scope of work and clearly defined deliverables which includes cell banking, scaling up production, purification, bioanalytics, and final finish and fill.