What are isotype controls?
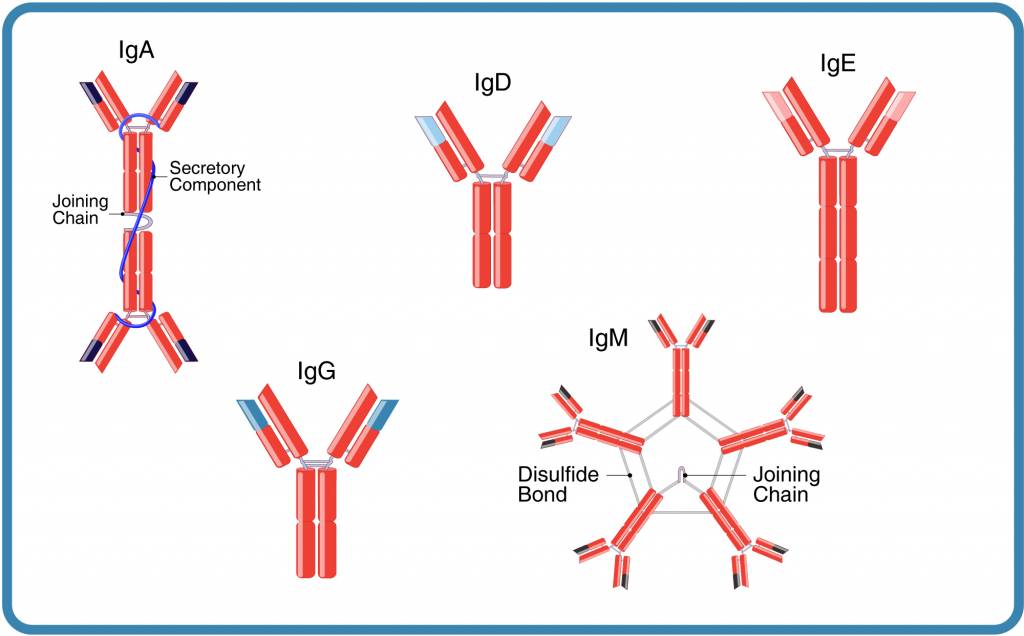
Figure 1. The five immunoglobulin classes or isotypes of immunoglobulins
An isotype control is an antibody selected as a negative control to detect non-specific binding in antibody-based experiments.
Understanding antibody structure is important for understanding how to choose an isotype control. Antibodies (immunoglobulins, Ig) are composed of two heavy chains and two light chains. In humans, there are five heavy chain isotypes α,δ,γ,ε,μ, corresponding to five antibody isotypes: α (IgA), δ (IgD), ε (IgE), γ (IgG), and μ (IgM).
In addition to the heavy chains, an antibody has one of two light chain isotypes, κ and λ, that may also play a role in the physicochemical properties of antibodies (Figure 1; references 1, 2).
A monoclonal isotype control is a negative control antibody from the same species, immunoglobulin class, subclass, and light chain as the primary antibody used in a particular scientific application.
Isotype control antibodies are developed in a similar way to primary monoclonal antibodies. Unlike primary antibodies, the immunogens are unrelated to like-proteins or epitopes present in the target organism or species. This avoids unwanted antibody-isotype control antigen interactions. It is important that the isotype and conjugate (e.g. FITC, PE, Biotin, Unconjugated) of the control matches the isotype and conjugate of the primary antibody exactly. The isotype of a particular antibody is usually specified by the supplier.
Why is it important to use isotype controls?
Primary monoclonal antibodies can interact both specifically and non-specifically with Fc receptors on cells, blood proteins, cellular proteins, carbohydrates, lipids, and tissues, which can lead to non-specific binding and misleading experimental results.
The appropriate isotype control antibody is therefore used to ensure the generation of reliable data in many applications, including:
- In vivo: pre-clinical animal studies requiring functional antibodies
- In vitro: assays such as flow cytometry, immunohistochemistry, fluorescent immunocytochemistry, western blotting, ELISA, and Luminex® Multiple Assays
The focus here is on in vivo applications.
How to Select Isotype Controls for in vivo Studies
There are a few important points that should be considered to ensure that isotype controls can differentiate non-specific signals from a positive signal, ensuring that the experimental result is generated by the primary monoclonal antibody and is not a false positive or false negative:
- Match the isotype control antibody to the same host species, class, and subclass (e.g., hIgG1, hIgG2, hIgG3, or hIgG4) as the primary antibody being used (Table 1). It is also desirable to match the light chain, which may affect the physicochemical properties of antibodies (1, 2).
- Isotype controls and primary antibodies should be of in vivo functional grade, selected based on purity specifications such as low endotoxin level, low presence of aggregates, no detectable leached protein A, and other contaminants that might cause false negatives or positives during preclinical studies.
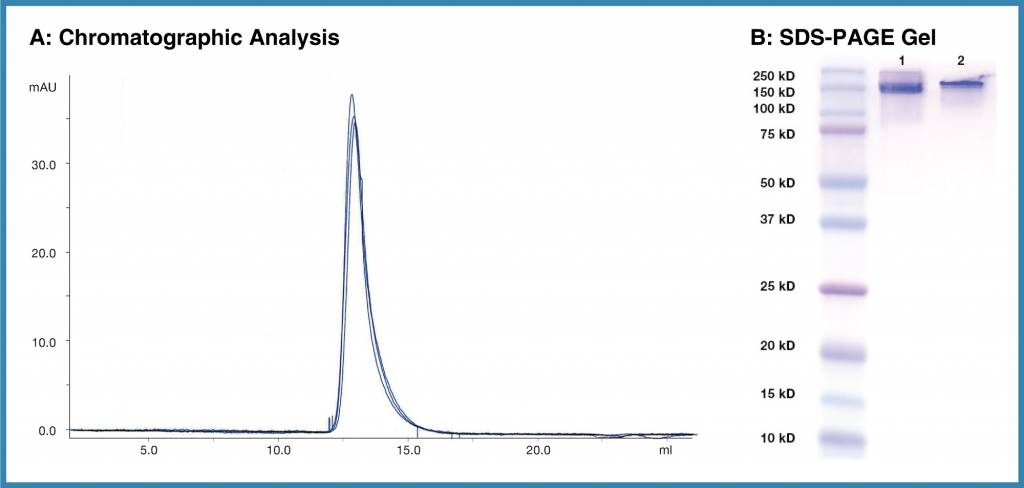
Figure 2. Purity and reproducibility of Leinco Isotype controls. A, Overlay of chromatograms representing three different lots of a Leinco isotype control. B, Two separate lots of Human IgG1 Isotype Control (Clone MOPC21.1, Lanes 2 and 3), further illustrating the purity and reproducibility of Leinco antibodies.
Examples of in vivo Functional Grade Primary Antibodies and Matching Isotype Controls
| Target | Primary Antibody Clone | Isotype |
| PD-1 | RMP1-14 | Rat IgG2a |
| Interleukin-1 R | JAMA-147 | Armenian Hamster IgG |
| CD151 | 50-6 | Mouse IgG1 |
| CD96 | 3.3 | Rat IgG1 |
It may be sufficient to use control antibody products that are produced from normal serum obtained from animals that have not been previously immunized.
Use of an Isotype Control in an in vivo Experiment
Figure 3. The ability of two in vivo functional-grade anti-PD-1 blocking antibodies (clones RMP1-14 and 29F.1A12, both isotype rat IgG2a κ) to reduce tumor size in a CT26 syngeneic model was tested compared to an isotype control (rat IgG2a κ). Testing involved groups of eight mice, each dosed at 10 mg/kg (0 for PBS control) per intraperitoneal administration, with twice-weekly (BIW) dosing for three weeks. Both anti-PD-1 clones were efficacious.
Isotype Controls in Analytical Assays and Flow Cytometry
While isotype controls are used in flow cytometry, they should be used with care to avoid variations compared to the primary antibody, including specificity, concentration, degree of aggregation, and fluorophore:antibody (F/P) ratio (3, 4).
As in in vivo applications, the isotype control antibody should match the primary antibody in terms of host species and class. In addition, the following points should be considered to ensure the generation of accurate and reproducible data:
- If the primary monoclonal antibody is conjugated with a fluorescent or enzymatic reporter molecule, the isotype control antibody should be conjugated with the same reporter molecule and at the same fluorophore or reporter:antibody ratio (F/P ratio).
- Purchase the isotype from the same supplier as the primary antibody as the F/P ratio can vary between suppliers.
- Use the same testing conditions when making comparisons, for example, antibody concentration, incubation times and temperatures, blocking and washing conditions, and detection methods.
Find out more about the range of isotype controls available from Leinco Technologies.
References
- Townsend CL, et al. Significant differences in physicochemical properties of human immunoglobulin kappa and lambda CDR3 regions. Front Immunol. 2016 Sep 27;7:388. doi: 10.3389/fimmu.2016.00388. PMID: 27729912; PMCID: PMC5037968.
- Montaño RF, and Morrison SL. Influence of the isotype of the light chain on the properties of IgG. J Immunol. 2002 Jan 1;168(1):224-31. doi: 10.4049/jimmunol.168.1.224. PMID: 11751966.
- Maecker HT, and Trotter J. Flow cytometry controls, instrument setup and determination of positivity. Cytometry Part A 2006; 69A:1037–1042
- Herzenberg L, et al. Interpreting flow cytometry data: A guide for the perplexed. Nature Immunology 2006; 7:681-685

