The Value of Low-Endotoxin Functional Antibodies & Isotype Control Specificity in Pre-Clinical Validation
Introduction
Low-endotoxin, high-quality functional antibodies are redefining how biologic medicines advance from concept to clinic. These critical tools enable biologics researchers to advance assay development faster, conduct more consistent comparative studies, and drive higher scientific rigor. But when endotoxins are lurking in preclinical functional antibodies, even the best-designed studies can be thrown off course by unreliable data and compromised drug candidates.
Research-grade Biosimilars are powering biologics research and development
Biosimilars are biological medicines that are designed to mimic an approved biologic drug, the reference product, commonly a monoclonal antibody (mAb). Biologics research models ranging from simple cell lines to complex, humanized mouse systems rely on biosimilars as real-world analogs in performance and hypothesis testing. With known safety, efficacy, and immunogenicity profiles, biosimilars lower development risks by providing benchmarks to demonstrate comparable performance of a new biologic drug in three fundamental parameters: 2, 3, 4, 5
- Pharmacokinetics (PK) and pharmacodynamics (PD) to demonstrate assay performance and bioequivalence
- Potency and structure-function correlation to study how molecular differences impact biological function
- Immunogenicity to calibrate anti-drug antibody (ADA) detection assays, measure cross-reactivity, and demonstrate that immunogenicity profiles fall within the expected range
Endotoxins can alter the performance of antibody function, causing false interpretation in biologic drug studies
Even when an antibody meets structural and functional comparability requirements, eliminating microbial contamination may not be enough to assure purity and safety. High batch rejection rates of biopharmaceuticals are often attributed to toxic or pyrogenic byproducts such as endotoxins.5

Endotoxins are lipopolysaccharides on the outer cell membrane of Gram-negative bacteria that are shed when the bacteria are growing, dividing, or dying. Even in small amounts, endotoxins can trigger cytokine storms, immune activation, or systemic inflammation leading to fever, shock, or organ failure. 6, 7 They cannot be detected by microbial sterility tests and are notoriously resistant to destruction or elimination, even by filtration and steam sterilization. In biosimilars R&D, endotoxins are prevalent byproducts of the E. coli-based systems often used to synthesize the biosimilar proteins. Even minuscule amounts of endotoxin contamination can lead to false-positive signals.7 With potentially life-threatening impact and difficulty to detect or eliminate, they are one of the most insidious threats in biosimilar development.
Endotoxin contamination is often characterized by spiking the test system with the endotoxin and then comparing recovery from the biosimilar and reference product. Recovery from the biosimilar that is significantly lower than expected (low endotoxin recovery; LER) might be caused by either poor correlation of the biosimilar and reference product or endotoxin masking in the biosimilar. Poor correlation may justify rejection of the biosimilar, whereas endotoxin masking can cause incorrect estimation of contamination, resulting in false positive or false negative results.5, 12, 13 Bound endotoxin can also cause safety and efficacy issues from adverse immune responses, impaired activity, undesirable off-target effects, altered metabolism, or diminished availability of the biologic.14
Multiple biochemical properties of endotoxins enable them to bind or form aggregates with other endotoxins or proteins, which can be difficult to detect and cause LER from the spiked sample.
| Causes of endotoxin masking in biosimilar R&D |
|---|
| Amphiphilic properties enable non-specific binding to positively charged or hydrophobic regions on mAbs. |
| Endotoxin-antibody binding can block true polyspecific binding sites or change the mAb tertiary conformation. |
| Endotoxin aggregates can induce mAbs to aggregate. |
| Endotoxins can organize into micelles or vesicles that inhibit detection. |
| In cell-based tests, endotoxins can stimulate cells, leading to non-specific uptake or binding that appears to reflect cell activation or specificity rather than true polyreactivity. |
LER requires rigorous control strategies and sophisticated testing methods to ensure accurate interpretation of analytical data and safety risks.
Endotoxin-mAb complexes can also confound interpretation of mAb polyspecificity and polyreactivity. Impaired binding to desirable targets can cause underestimation of polyspecific biosimilar availability. Non-specific binding to unintended targets can overestimate polyspecificity with false potential for new biosimilar activity. 15, 16 Identification and analysis of endotoxin-associated polyspecificity and polyreactivity are vital to ensure endotoxins are not masking true effects of the functionality, reducing reproducibility, and delaying readiness for approval.
Mouse models are vital to understanding potential clinical impact of endotoxemia
Unlike small molecule generic drugs, biosimilars are produced in inherently variable living systems. Therefore, they are not chemically identical to the reference product. Even small changes in molecular structure or stability can launch a dangerous immune response. To ensure patient safety with these novel medicines, the FDA, European Medicines Agency (EMA), and Japanese Pharmaceuticals and Medical Devices Agency (PMDA) have all established regulations defining the critical quality attributes that must be met to demonstrate comparable performance of a biosimilar with its reference product.8, 9 Details vary by country, but in general, researchers must demonstrate similarity with very high reproducibility across a broad range of characteristics including structure, specificity, potency, physiochemical and biochemical properties, safety, purity, and route of administration.7
In preclinical mouse models, endotoxemia is often used to study acute inflammatory responses that are similar to sepsis and systemic inflammation in humans. These early indicators include lethargy, weight loss, and acute cytokine release.17 Mouse models have revealed potentially severe effects of endotoxemia associated with several symptoms of sepsis, including heart rate variability,18 acute lung injury,17 cardiac fibrosis,14 nervous system hyperactivation,14, 19 and organ dysfunction.14
Endotoxin immune response is dose dependent.6, 7, 9 Under FDA regulations, the limit of endotoxin (measured in endotoxin units, “EU”) allowed for any injectable pharmaceutical product is set to the concentration at which pyrogenicity is determined, currently 5 EU/kg body weight/hour.7, 10 For example, in a typical 25-gram mouse, a biosimilar dose of 5 mg/kg contaminated with 1 EU/mg of endotoxin meets the maximum allowed amount in a single injection. Levels above 0.1 EU/mg can induce T cell proliferation and CD14+ myeloid cell expansion.7, 10 Investigational biologics tested as combination therapies to identify multidrug regimens present additional risk. These protocols often require administering multiple biologics within a close timeframe. Even if each product contains only small amounts of endotoxins, accumulation from multiple sources can lead to dangerous levels.6, 9

Leinco Technologies research-grade biosimilars exhibit endotoxin levels comparable to pharmaceutical-grade product
Leinco Technologies has spent over 30 years advancing quality control processes for optimization of research-ready biosimilars. Overlapping SDS-polyacrylamide gel electrophoresis and size-exclusion chromatography tests from multiple production lots confirm these biosimilars are strong analogs to their reference products in size, purity, and structure. Functional assays demonstrate highly reproducible performance similarity with the reliability of animal-free manufacturing systems. Leinco Technologies isotype controls, used to test biosimilar endotoxin levels, are designed and tested to the same stringent criteria.
FDA guidelines specify that the reagents used in LAL endotoxin detection methods must be obtained from an FDA-licensed manufacturer and must be designed specifically for the method chosen.7, 10 Every lot of Leinco Technologies’ biosimilars is tested on the Pyros Kinetix Flex system (Associates of Cape Cod, Inc.) using FDA-licensed LAL reagents for endotoxin detection. The Associates of Cape Cod (ACC) Pyros Kinetix Flex system is compliant with the requirements of the FDA, USP, and European Pharmacopoeia (Ph. Eur.) for conducting the Bacterial Endotoxins Test (BET) on raw materials, in-process samples, and final drug products. This rigorous screening demonstrates endotoxin levels less than 0.1 EU/mg (Table 1) in typical batches, as recommended for in vitro immunogenicity assays to ensure accurate and reliable data.7
| Leinco Technologies Antibody | Antibody concentration (mg/mL) | Endotoxin (EU/mg) |
|---|---|---|
| anti-KLH* HKSP84 P382 | 3.9 | .063 |
| anti-BTV** HKSP M1411 | 10.2 | .036 |
| Leinco Palivizumab | 7.3 | .010 |
Table 1: The actual endotoxin concentrations in three Leinco Technologies antibodies were measured and are represented here, determined using the Endosafe® Nexgen-PTS™ system with an LAL cartridge. These results show endotoxin levels 10X below our specifications.
*KLH – Keyhole limpet hemocyanin
**BTV – Bluetongue virus

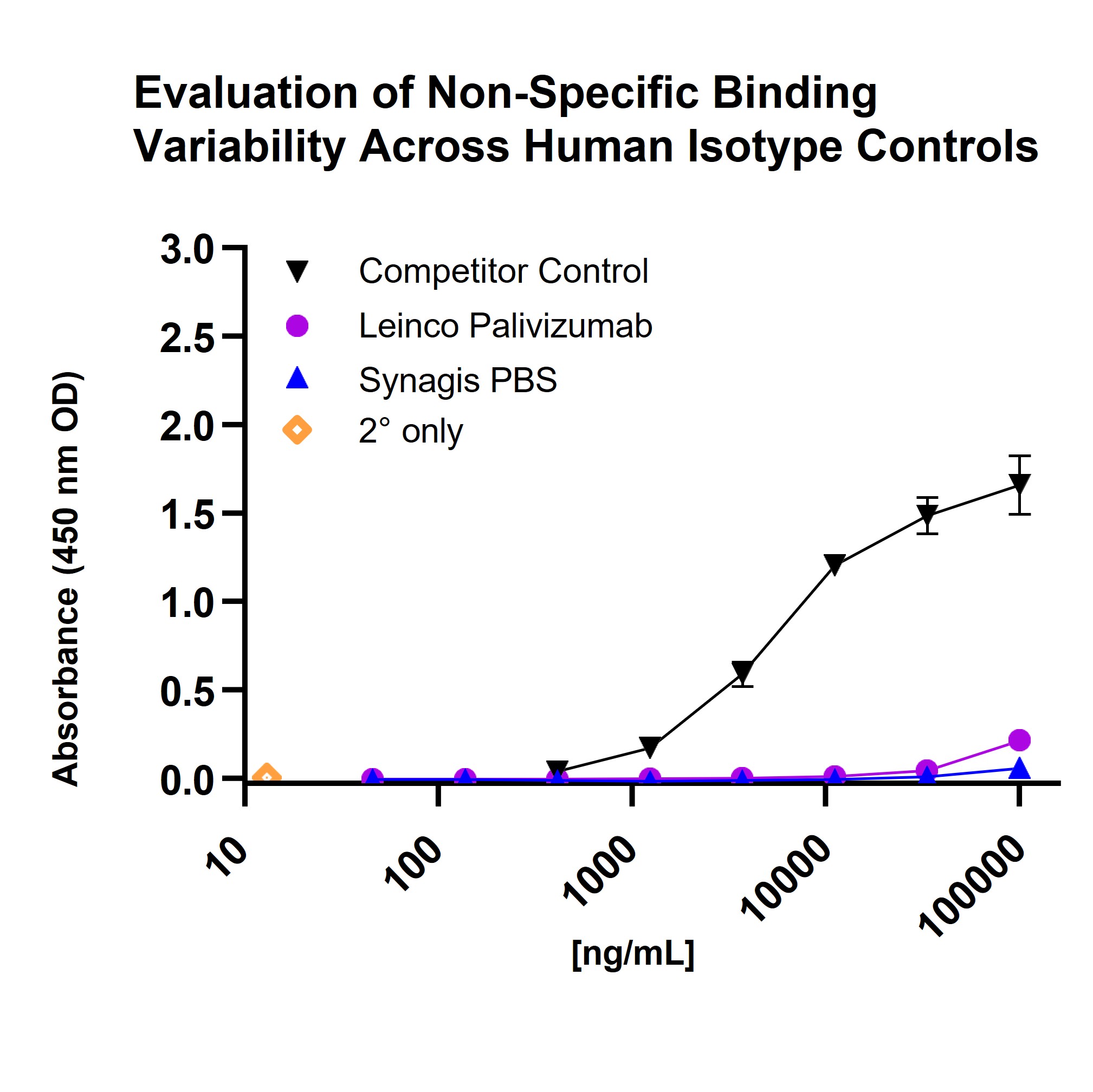
Figure 1. Non-specific binding profile of Leinco Biosimilar Palivizumab (Anti-RSV F Protein).The chart demonstrates that Leinco Biosimilar Palivizumab, anti-RSV F Protein (magenta circles), exhibits consistently low non-specific binding across all tested concentrations, with absorbance values nearly identical to those of PBS and secondary-only controls. In contrast, the Competitor Control displays a pronounced increase in non-specific binding at higher concentrations, indicating Leinco’s product offers superior assay non-specificity.
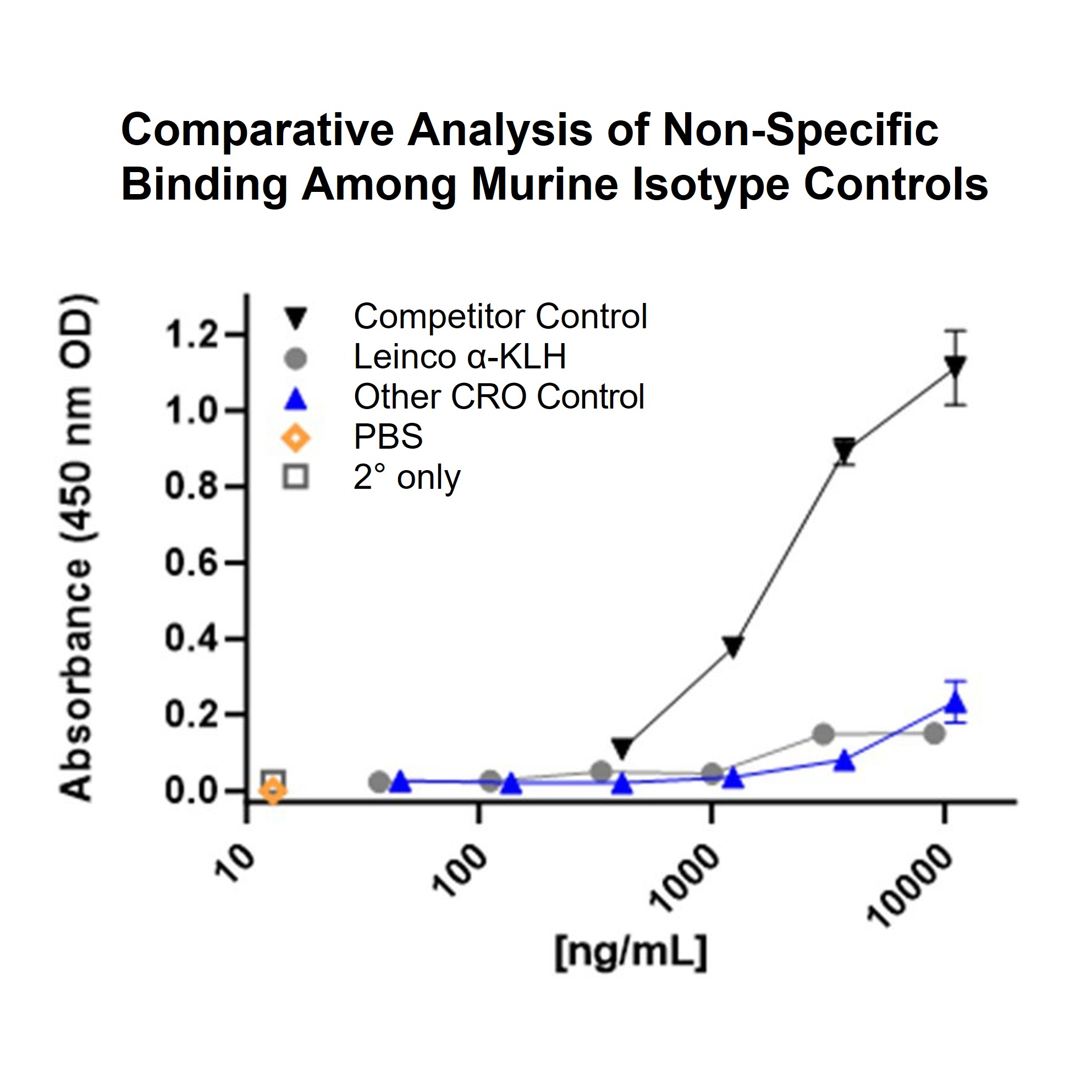
Figure 2. Comparative analysis of non-specific binding: Leinco Mouse IgG1 Isotype Control (Anti-KLH) vs. competitor. The chart shows that the Leinco Mouse IgG1 Isotype control (Anti-KLH, gray circles) demonstrates consistently low non-specific binding across all concentrations tested, with absorbance values near those of buffer and secondary-only controls. In comparison, the Competitor Control exhibits much higher non-specific binding at increased concentrations, indicating Leinco’s isotype control offers superior assay non-specificity.
Conclusion
This study demonstrates that maintaining extremely low non-specific interaction in animal studies is crucial for reliable data interpretation and candidate selection. The measured results confirm product quality standards for preclinical functional antibodies such as purity, endotoxin concentration, and batch consistency, by directly influencing the accuracy of screening assays and the validity of immunogenicity and efficacy determinations. Even trace amounts of endotoxin contamination can result in false negative or false positive findings, jeopardizing drug development by advancing flawed candidates or discarding promising ones prematurely.
The use of Leinco preclinical functional antibodies in this experimental setup was reinforced as an essential methodological safeguard. Isotype controls serve as negative controls to distinguish specific antibody-antigen binding from non-specific interactions in functional applications, especially those mediated by Fc receptors or caused by antibody cross-reactivity. Their application helps researchers determine whether observed signals reflect actual biological activity or are artifacts stemming from background noise, sample preparation, or reagent impurities. By consistently incorporating high-quality Leinco isotype controls, studies reduce experimental bias and enhance the robustness and reproducibility of data, leading to more confident conclusions and supporting regulatory compliance in biosimilar product characterization. Researchers are strongly encouraged to adopt stringent isotype control practices in all antibody-based assays to ensure that data interpretation remains accurate, reproducible, and scientifically valid.
Are you ready to upgrade your next in vivo biologics study or translational assay? Explore catalog or custom functional antibody solutions optimized for your research needs.
References
- Winegarden W (2024) Biosimilars Often Reduce Prices by 50 Percent or More. The Biosimilar Promise (Pacific Research Institute) 1:1-3 (October 2024). https://www.pacificresearch.org/biosimilars-often-reduce-prices-by-50-percent-or-more
- Shibata H, Harazono A, Kiyoshi M, et al. (2025) Characterization of Biosimilar Monoclonal Antibodies and Their Reference Products Approved in Japan to Reveal the Quality Characteristics in Post-approval Phase. BioDrugs. 39(4):645-667. doi: 10.1007/s40259-025-00722-4
- Ishii-Watabe A, Kuwabara T (2019) Biosimilarity assessment of biosimilar therapeutic monoclonal antibodies. Drug Metabolism and Pharmacokinetics 34(1):64-70. doi: 10.1016/j.dmpk.2018.11.004
- Iskit AB. (2025) Biosimilars and interchangeability: Regulatory, scientific, and global perspectives. Eur J Pharm Sci. 213:107224. doi:10.1016/j.ejps.2025.107224
- Williams, KL (2019) The Biologics Revolution and Endotoxin Test Concerns. In: Williams, K (eds) Endotoxin Detection and Control in Pharma, Limulus, and Mammalian Systems. Springer, Cham. doi: 10.1007/978-3-030-17148-3_8
- Pharmaceutical Technology Europe (2009) Removing endotoxin from biopharmaceutical solutions. PharmTech.com (online article, Oct 1, 2009). Accessed Sept. 30, 2025. https://www.pharmtech.com/view/removing-endotoxin-biopharmaceutical-solutions
- Jeong YH, Lennon G, Veldman G, et al (2025) Establishing endotoxin limits to enhance the reliability of in vitro immunogenicity risk assessments. MAbs. 17(1):2458627. doi:10.1080/19420862.2025.2458627
- Charles River Laboratories (2025) Guide to USP Chapter <86> for Bacterial Endotoxin Testing (webpage). Accessed Oct. 1, 2025. https://www.criver.com/resources/usp-chapter-86
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Veterinary Medicine, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health, U.S. Department of Health and Human Services FDA Office of Regulatory Affairs (2012) Guidance for Industry Pyrogen and Endotoxins Testing: Questions and Answers. Published June 2012. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-pyrogen-and-endotoxins-testing-questions-and-answers?utm_source=chatgpt.com
- Department of Health, Education, and Welfare, Public Health Service, FDA *ORA/ORO/DEIO/IB* (2014) Bacterial Endotoxins/Pyrogens. Published Nov. 17, 2014. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/bacterial-endotoxinspyrogens
- Charton E (2022) European Pharmacopoeia Approach to Testing for Pyrogenicity. Am Pharma Rev July/Aug 2022:18-22.
- Williams K (2014). Endotoxin Test concerns of biologics part II: Developing New tools. Am Pharma Rev Endotoxin Supp 2014 17(2):16-20.
- Wespel M, Geiss M, Nägele M, et al. (2022) The impact of endotoxin masking on the removal of endotoxin during manufacturing of a biopharmaceutical drug product. J Chromatogr A. 1671:462995. doi:10.1016/j.chroma.2022.462995
- Goossens E, Li J, Callens C, et al. (2022) Acute Endotoxemia-Induced Respiratory and Intestinal Dysbiosis. Int J Mol Sci. 23(19):11602. doi:10.3390/ijms231911602
- Éliás S, Wrzodek C, Deane CM, et al. (2024) Prediction of polyspecificity from antibody sequence data by machine learning. Front Bioinform 3:1286883. doi:10.3389/fbinf.2023.1286883
- Ritzén U, Rotticci-Mulder J, Strömberg P, et al. (2007) Endotoxin reduction in monoclonal antibody preparations using arginine. J Chromatogr B Analyt Technol Biomed Life Sci 856(1-2):343-347. doi:10.1016/j.jchromb.2007.06.020
- Rojas M, Woods CR, Mora AL, et al. (2005) Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol 288(2):L333-L341. doi:10.1152/ajplung.00334.2004
- Fairchild KD, Saucerman JJ, Raynor LL, et al. (2009) Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. Am J Physiol Regul Integr Comp Physiol 297(4):R1019-R1027. doi:10.1152/ajpregu.00132.2009
- Shemer A, Scheyltjens I, Frumer GR, et al. (2020) Interleukin-10 Prevents Pathological Microglia Hyperactivation following Peripheral Endotoxin Challenge. Immunity 53(5):1033-1049.e7. doi:10.1016/j.immuni.2020.09.018
- Charles River Laboratories (2025) Endosafe nexgen-PTS (webpage). Accessed Sept 30, 2025. https://p.widencdn.net/bfsnx1/MS-TS-nexgen-pts-regulatory-requirements-uspep

