CHO Bioproduction Services
Supercharge production of your antibodies and other recombinant proteins
Are you looking for a reliable and efficient way to produce antibodies and other recombinant proteins? While there are numerous factors to consider when choosing an expression system, if you are looking for efficiency, quality, scalability, and a low cost per mg of antibody, look no further than our new CHO GS-/- expression system.
Boost productivity with Leinco’s CHO GS-/- expression system
When developing recombinant antibodies and other proteins, the choice of expression system can profoundly impact process titers, downstream timelines, regulatory submission success, and the economic viability of your development strategy.
Expression systems that use glutamine synthetase (GS) as a selectable marker are increasingly preferred for production of biotherapeutics and diagnostics due to shorter timelines to achieve sufficient titers for large-scale manufacturing.
Leinco combines the advantages of a robust CHO GS-knockout cell line with the power of UCOE technology, which enhances gene expression by creating a transcriptionally active environment around the inserted transgene. Together, these elements shorten the turnaround time to produce your protein, while maximizing the yield.
In addition, with Leinco’s new CHO Cell Line Development service, high-performing clonal pools can be banked and taken for forward for stable cell line development. This seamless approach paves the way for faster and more efficient translation from the bench to clinical trials and commercial production.
Key Benefits
Why CHO Cells
As the workhorse of the biopharmaceutical industry, Chinese Hamster Ovary (CHO) cells have been the gold standard for antibody production for decades.
Mammalian cell lines are used to manufacture over half of all recombinant protein and monoclonal antibody (mAb)-based biopharmaceuticals licensed for US and EU markets. Of these, about 80% are produced in CHO cell lines. 1
CHO cells have a well-established regulatory history. With the ability to adapt to suspension culture, they are readily scaled for high volume production in bioreactors. Since CHO cell lines are derived from rodents, they have a lower risk of propagating human viruses compared to human expression hosts. They also perform human-like post-translational modifications important for protein stability, function and safety in clinical applications.
1Walsh G and Walsh E. Biopharmaceutical benchmarks 2022. Nat Biotechnol (2022) 40(12):1722-1760.
Higher process titers, shorter timelines
Transient transfection is a rapid and economical method for recombinant protein production, but may not always provide sufficient titers for your application because episomal plasmid DNA is degraded or diluted over time. Our optimized expression system, based on glutamine synthetase (GS) selection, affords the option of applying selective pressure in culture, so that only cells with integrated plasmid DNA survive the selection process.
To further enhance yields and reduce development timelines, Leinco takes advantage of ubiquitous chromatin opening element (UCOE) technology to prevent epigenetic silencing of your gene of interest (GOI). This increases the number of high-expressing cells per transfection, enhances transgene expression stability, and shortens the development process.
While a selection-based strategy can be lengthier than transient transfection, it results in stable recombinant cell pools that are readily scaled to produce gram quantities of your protein. Optionally, top-performing pools can be banked and taken forward for stable cell line generation.
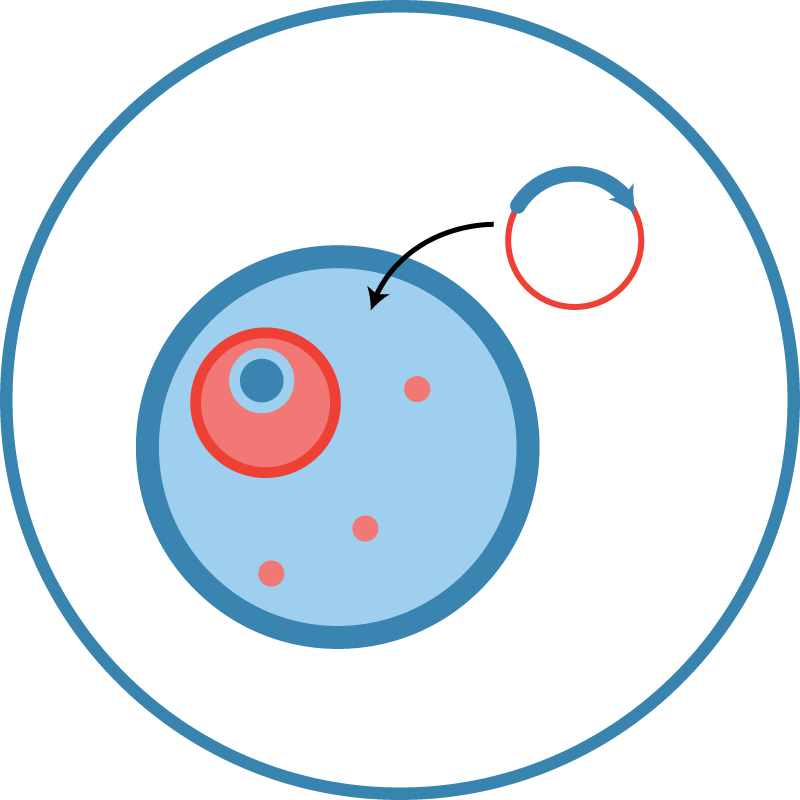
CHO GS-knockout cell line (CHOZN® GS-/-)
The cell line at the heart of Leinco’s expression system is a CHO-K1 variant that has been genetically engineered to delete both alleles of the GS gene. To survive selection in glutamine-free media, the transfected cells must integrate plasmid DNA encoding the GS enzyme.
Unlike traditional CHO cell lines, CHOZN® GS-/- cells produce no endogenous GS. This allows selection without addition of the GS inhibitor methionine sulfoximine (MSX), which can negatively impact cell growth and cannot be used in drug production.
The CHOZN® GS-/- cell line has a long track record of successful use in industry. It offers all the benefits of a traditional CHO cell line, while contributing to higher yields and a shorter time to results.
Figure 2. UCOE® Expression Vector. UCOE elements are incorporated into the vector upstream of light chain and heavy chain cassettes for expression of the GOI under the control of powerful hCMV promoters.
UCOE technology
The process of selection and enrichment for high-expressing recombinants can be time-consuming and laborious. Since plasmid DNA integrates randomly into the genome, there is a high chance that the gene of interest (GOI) will integrate into a region that is transcriptionally inactive (heterochromatin) or prone to epigenetic silencing.
UCOEs are methylation-free DNA regulatory elements that have evolved to prevent epigenetic gene silencing. When integrated into heterochromatin, they resist methylation of nearby genes, effectively holding the DNA in an open configuration accessible to transcription machinery.
The UCOE® expression vector increases the number of high-expressing clones in transfected populations, for higher titers and shorter development times.
One system for seamless development
The UCOE® expression vector was designed to perform optimally with the CHOZN® GS-/- cell line and chemically defined GS selection media. The complete expression system provides a robust and seamless solution—suitable for upstream development, stable cell line generation, and scale-up for industrial manufacture.
A collaborative process
From project definition through to production and release testing of your antibody or other recombinant protein, our specialist teams work closely with you to ensure high-quality results and timely delivery.
How it Works