Novel SARS-CoV-2 Neutralizing Antibody Assay
A Complete Overview of the Development, Validation, & Interpretation of SARS-CoV-2 High-throughput Neutralization Assay Results
Abstract
Given the global health emergency and COVID-19 pandemic, the need for rapid development of effective vaccines and therapies is at an all time high. Developing an effective vaccine requires an understanding of the adaptive immune response to SARS-CoV-2. An assay to measure circulating antibodies, specifically neutralizing antibodies (NAbs) that disrupt receptor-binding domain (RBD) and angiotensin-converting enzyme 2 (ACE2) binding to prevent SARS-COV-2 cell entry would be an important research tool.
Leinco Technologies has extensive experience manufacturing recombinant antibodies and proteins. In early February, we started developing and manufacturing SARS-CoV-2 recombinant antibodies sequenced from plasma B cells of COVID-19 survivors, along with SARS-CoV-2 recombinant proteins. Most notable, we developed a novel SARS-CoV-2 neutralizing test that qualitatively detects and ranks anti-SARS-CoV-2 RBD NAbs of all isotypes in plasma or serum.
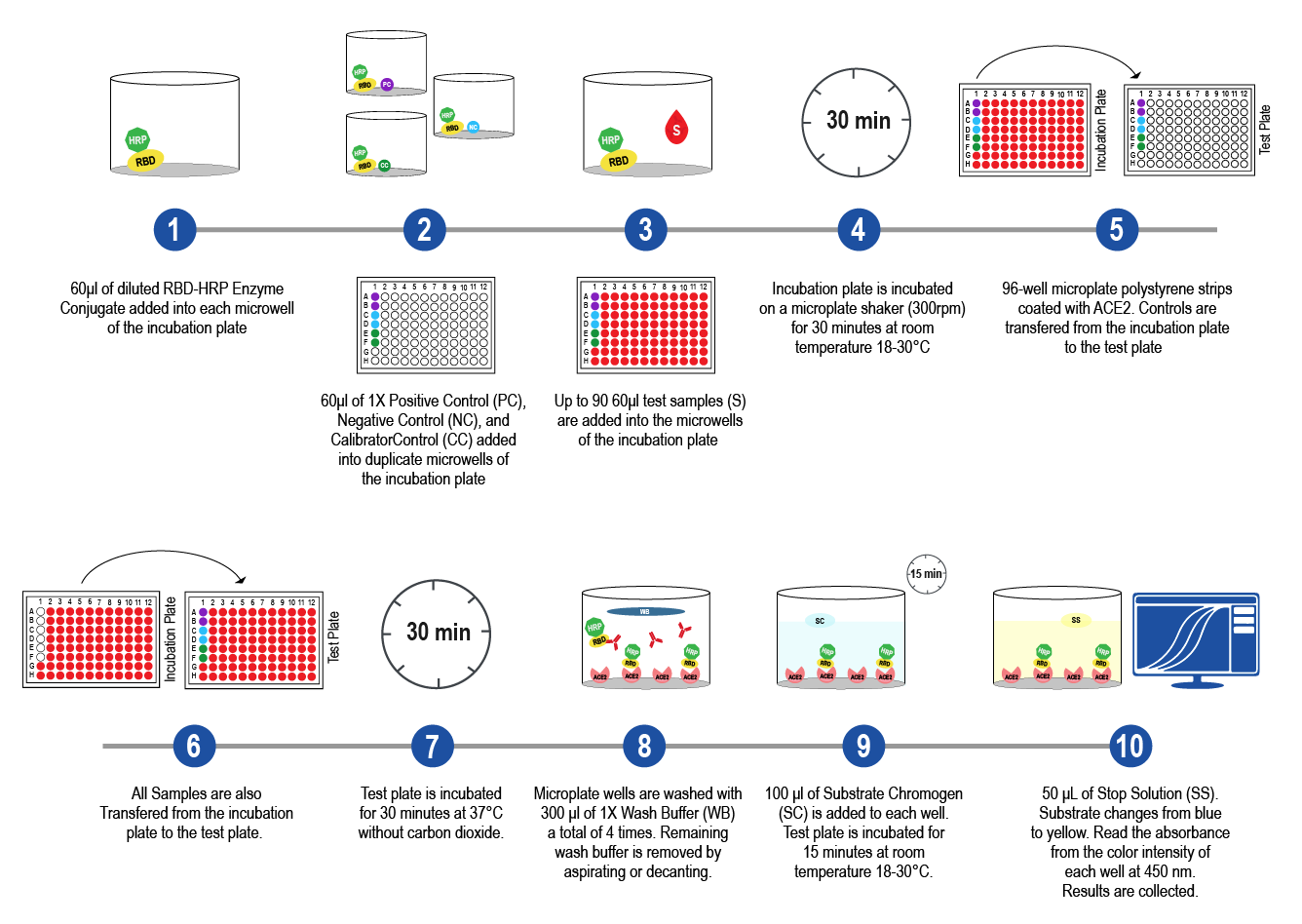
The ImmunoRankTM neutralization MICRO-ELISA compares to a live virus neutralization test, such as the Plaque Reduction Neutralization Test (PRNT) and Focus Reduction Neutralization Test (FRNT), but is less laborious, takes only 80 minutes to complete, and does not require a BSL3 laboratory. Current tests for measuring the presence of neutralizing antibodies are not amenable to high-throughput screening and are not widely available in clinical laboratories. Leinco urgently solves this problem by bringing ImmunoRankTM to the market; a high-throughput, easy-to-use, SARS-CoV-2 neutralization test. ImmunoRankTM identifies convalescent plasma with potent anti-SARS-CoV-2 NAbs for efficiently treating patients with COVID-19. Within this white paper, Leinco discusses the ImmunoRankTM assay principles, procedures and workflow, validation, and interpretation of data.
Download
White Paper
[gravityform id=”11″ title=”false” description=”true”]