Overcome ascites production headaches: in vitro bioreactors deliver high yield, quality mAbs
Monoclonal antibodies (mAbs) are powering breakthroughs that reshape diagnostic testing with new approaches to biomarker detection, tissue and cell imaging, assay development, and companion diagnostics. How mAbs are produced can make a difference in the speed, efficiency, and quality of test development.
Two approaches to mAb production: In vivo ascites and in vitro bioreactors
Producing mAbs in the ascites fluid of live mice was the standard method until the 1990s, but the distress it causes the mice has led to ethical concerns. The method is now prohibited for most applications.1 Advancements in in vitro bioreactor technologies, supported by animal-origin-free media, that perform comparably to the ascites method are welcome alternatives in the effort to reduce animal use.
How do these two methods work?
In vivo mAb production in mice
- A priming adjuvant is injected into the peritoneal cavity to stimulate production of ascites fluid.
- Hybridoma cells are introduced and grow to become an ascites tumor, which releases mAbs into the ascites fluid.
- The mAb-enriched ascites fluid is periodically harvested from the mouse using a needle or catheter and then clarified to remove debris for downstream purification.
In vitro mAb production in a bioreactor
-
- Antibody-producing hybridoma cells are transferred into the production vessel, which may be roller bottles, fed-batch tanks, perfusion tanks, or hollow fiber bundles. Researchers can decide whether to use conventional, serum-free, or animal-component-free medium.
- Cells proliferate and produce mAbs under controlled culture conditions.
- mAb-enriched supernatant is collected according to the bioreactor method and then clarified to remove debris for downstream purification.
In vitro bioreactors can match or exceed mAb yield from ascites
The ascites method was valued for generating high yields, but factors such as mouse immune responses and human error can diminish mAb generation. In contrast, in vitro bioreactors, provide a stable, nutrient-rich environment in which cells thrive and deliver high yields that are comparable, or even greater than, yields from ascites.1, 2, 3
In vitro bioreactors advance animal welfare initiatives
The 3Rs: First, Replace – don’t use animals if you have an alternative; second, Reduce – use as few animals as you can; and third, Refine – adapt protocols to improve the welfare of the animals you still use. This framework was created over 50 years ago as guidance for all users of animals in science to minimize use and suffering of the animals without compromising the integrity of their work.5
Eliminating the use of mice by switching from ascites systems to in vitro bioreactors is one of the most direct opportunities to advance your 3Rs initiatives. R&D labs can also reduce animal use well upstream of in vitro cell culture by converting to media that contains no animal-derived components. For example, Leinco Technologies’ AlphaGrow™ culture media are precisely formulated to support optimal hybridoma growth and mAb production with no animal-origin components.
In vitro bioreactors can simplify regulatory compliance
Ethical appreciation of this concept has led many countries to establish strict regulations that dictate how animals can be used in science. Using live animals for mAb production is almost entirely banned in North America, Europe, the United Kingdom, and Australia. Approval for specific applications often comes with stringent ethics protocols at both national and institutional levels.2, 5 The benefits of the ascites method might be lost against burdensome compliance requirements that can grind research to a near standstill. Every conversion of the ascites method to in vitro bioreactors frees researchers from these obligations.
In vitro bioreactors deliver high purity comparable to ascites
Accumulation of host cell proteins and other waste in cell cultures from animals can interfere with cell growth, yield, and purity. In vitro systems inherently eliminate several fundamental sources of impurities. Bioreactor media is carefully formulated with known serum and nutrients. During culture, parameters are optimized and controlled to maximize yield. With no mice, there are also no host cell proteins, cytokines, or other ascitic fluid contaminants. The result is high yield of high purity mAbs.1, 2, 3, 6 Eliminating animal-derived products from media can also reduce risk of pathogens and other extraneous material, simplifying purification steps and lab safety protocols.
In vitro bioreactor mAb production is more consistent than the ascites method
Ascites-based mAb production is inherently uncontrollable. Every mouse is a unique mAb production vessel. Naturally varying factors such as immune reactions, age, and genetics drive inconsistencies in yield and quality.7 Human error caused by differences in timing or technique introduces additional variability.
With closed, controllable, hands-off operation, in vitro bioreactors provide consistent yield and quality within batches and across multiple runs.2 Animal-origin-free medium with fixed, uniform composition also supports more reliable cell growth.
Scalability, flexibility, and control
Mice don’t scale easily. In early research, the ascites method might seem like a cost-effective way to produce mAbs. But as your initiative grows, every additional mouse must be housed and tracked. Additional expertise is required during mAb production. Costs can rise quickly.
Leinco Technologies’ in vitro bioreactor mAb production systems are designed for scalability. Controlled culture and minimal intervention replace animal care and handling. As production needs expand, culture and harvesting parameters can be tuned to optimize yield and quality at any scale.
Proof from production – a case study
Leinco Technologies collaborated with an in vitro diagnostics raw material provider to shift their established ascites production system to a more scalable solution using an in vitro bioreactor. With optimized cell lines, scaled bioreactor protocols, cell banks, and rigorous quality control, this customer now benefits from:
- Significantly reduced production costs
- Improved antibody consistency and quality
- Improved scalability with faster turnaround time
- Elimination of live animal systems
Conclusion
Trust the experts. In vitro bioreactors can produce mAbs with yield, purity, and consistency that is comparable or better than the ascites method. In vitro production and animal-origin-free culture media advance animal welfare standards, simplify scale-up, and streamline regulatory compliance. For a comprehensive solution to your mAb production needs, visit Leinco’s Monoclonal Antibody Manufacturing. Our advanced bioreactor, media technologies, and proven expertise ensure that you receive the highest quality antibodies with consistent lot-to-lot reproducibility and optimized production costs.
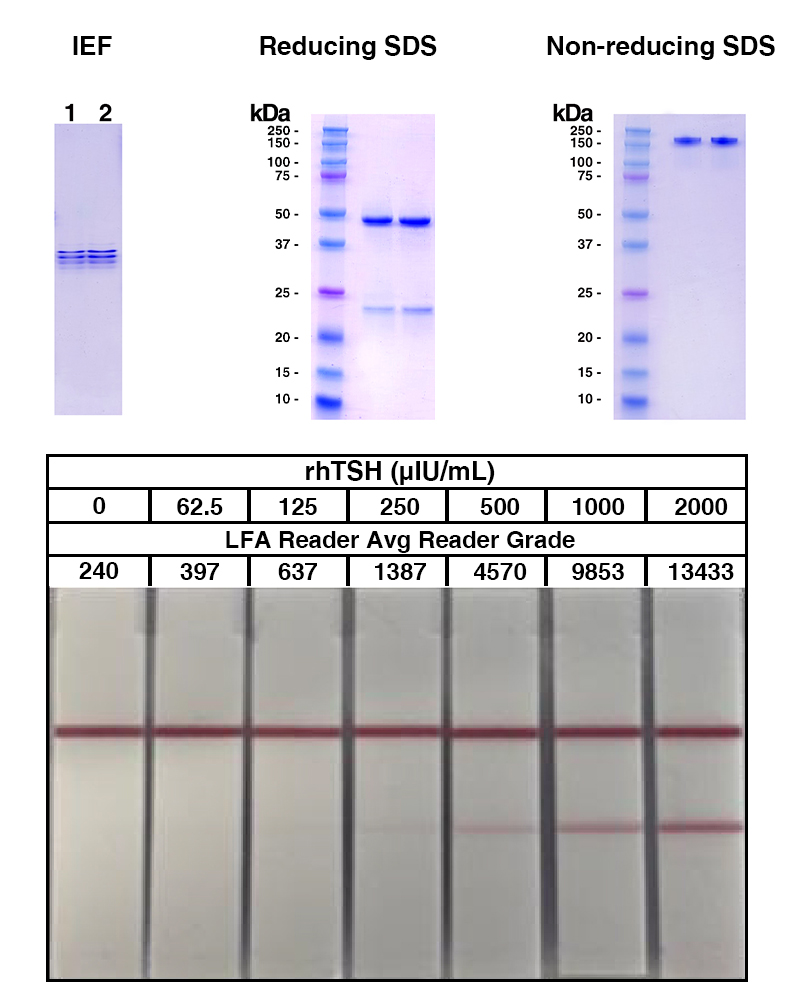
LFA Data compared to Gel Data
Lane 2 in the SDS-PAGE gel demonstrates the antibody produced through ascites (in vivo) method, while lane 3 displays the same TSH antibody clone generated via in vitro bioreactor technology. Both lanes show equivalent band patterns, confirming the molecular integrity and comparability between the two production approaches for this monoclonal antibody. Both antibody preparations were applied in lateral flow assays, demonstrating that the in vitro bioreactor-produced TSH antibody performs equivalently to the ascites-derived version.
References
- National Research Council (US) Committee on Methods of Producing Monoclonal Antibodies (1999). Monoclonal Antibody Production: 2, In Vitro Production of Monoclonal Antibody. National Academies Press (US) https://www.ncbi.nlm.nih.gov/sites/books/NBK100192/
- National Research Council (US) Committee on Methods of Producing Monoclonal Antibodies (1999). Monoclonal Antibody Production: 4, Summary of Advantages and Disadvantages of In Vitro and In Vivo Methods. National Academies Press (US) https://www.ncbi.nlm.nih.gov/sites/books/NBK100200/
- Liquidyne (2015) Hollow Fiber Provides a Sweet Spot for Several Biomanufacturing Applications. Cell Culture DISH (blog, September 15, 2015) Accessed September 23, 2025. https://cellculturedish.com/hollow-fiber-provides-a-sweet-spot-for-several-biomanufacturing-applications/
- Jackson LR, Trudel LJ, Fox JG, et al. (1996) Evaluation of hollow fiber bioreactors as an alternative to murine ascites production for small scale monoclonal antibody production. J Immunol Methods. 189(2):217-231. doi:10.1016/0022-1759(95)00251-0. https://pubmed.ncbi.nlm.nih.gov/8613673/
- Vasbinder MA, Locke P (2016) Introduction: Global Laws, Regulations, and Standards for Animals in Research. ILAR Journal 57(3):261–26. https://doi.org/10.1093/ilar/ilw039
- Lide Biotech (2023) In Vitro Models: Bridging the Gap Between the Lab and the Clinic (online blog, June 12, 2024) Accessed September 23, 2025. https://www.lidebiotech.com/beta/blog/vitro-models-bridging-gap-between-lab-and-clinic
- Brodeur BR, Tsang P, Larose Y (1984) Parameters affecting ascites tumour formation in mice and monoclonal antibody production. J Immunol Methods 71(2):265-272. https://pubmed.ncbi.nlm.nih.gov/6736661/

