Recombinant Human Epiregulin
Data
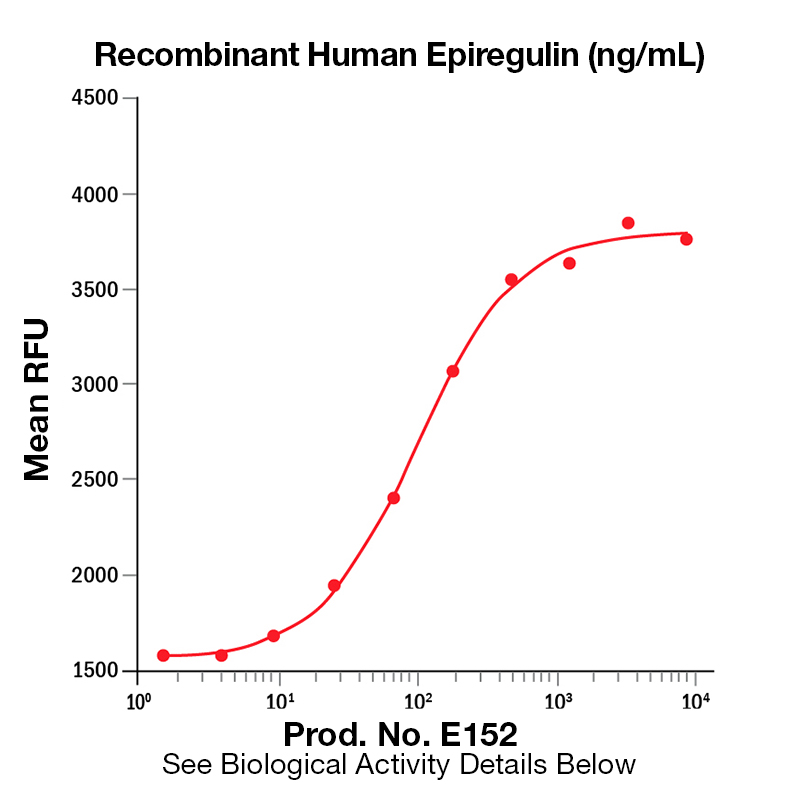
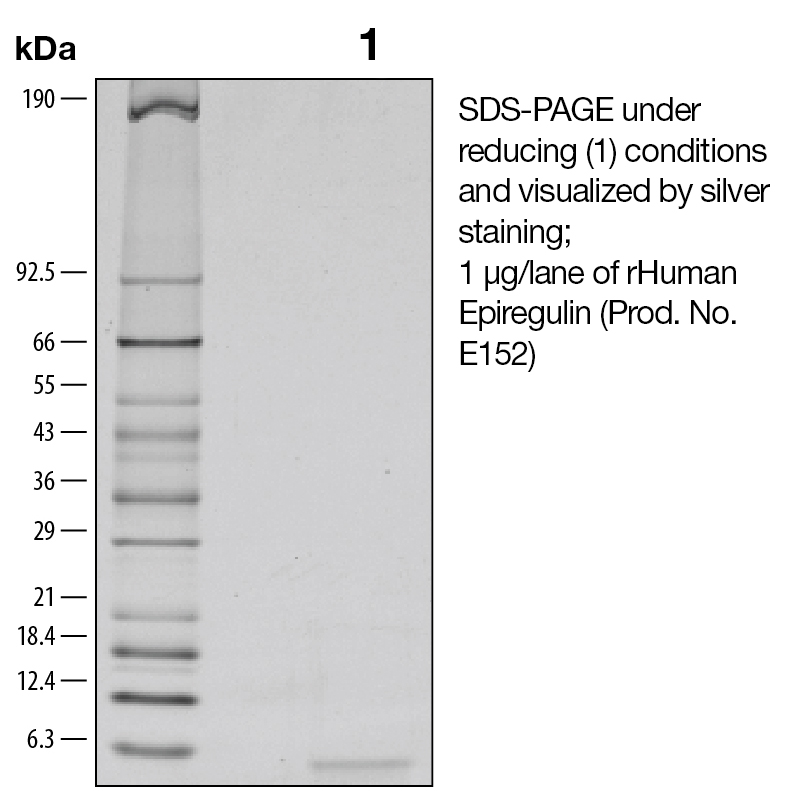
BackgroundEpiregulin (also known as EREG) is a member of the EGF family of growth factors. It binds specifically to EGFR (ErbB1) and ErbB4 but not ErbB2 or ErbB3 (1). It is expressed mainly in the placenta and peripheral blood leukocytes, as well as certain carcinomas of the bladder, lung, kidney and colon (2). EREG has been shown to act as an autocrine growth factor for human keratinocytes, stimulating the proliferation human keratinocytes under both subconfluent and confluent culture conditions in the absence of exogenous EGF family growth factors. In keratinocytes EREG upregulates the mRNA levels of HB-EGF, amphiregulin, and TGF-alpha. It has been shown that EGF, HB-EGF, amphiregulin, and TGF-alpha increase EREG mRNA levels (3). EREG inhibits the growth of several epithelial tumor cells and stimulates the growth of fibroblasts and various other types of cells. It binds to the EGF receptors of epidermoid carcinoma A431 cells much more weakly than EGF, but is much more potent than EGF as a mitogen for rat primary hepatocytes and Balb/c 3T3 A31 fibroblasts (4). EREG has been implicated in the implantation process during pregnancy (5). Protein DetailsPurity >97% by SDS-PAGE and analyzed by silver stain. Endotoxin Level <0.1 EU/µg as determined by the LAL method Biological Activity The biological activity of Human Epiregulin was determined by its ability to stimulate proliferation in a mouse fibroblast cell line, Balb/3T3 (Rubin, J.S. et al., 1991, Proc. Natl. Acad. Sci. USA 88:415). The expected ED<sub>50</sub> is typically 0.125 - 0.75 ng/mL. Protein Accession No. Amino Acid Sequence mvsitkcss dmngyclhgq ciylvdmsqn ycrcevgytg vrcehffl N-terminal Sequence Analysis Met State of Matter Lyophilized Predicted Molecular Mass The predicted molecular weight of Recombinant Human Epiregulin is Mr 5.4 kDa. Predicted Molecular Mass 5.4 Formulation This recombinant protein was 0.2 µm filtered and lyophilized from modified Dulbecco’s phosphate buffered saline (1X PBS) with BSA as a carrier protein. pH 7.2 – 7.3 with no calcium, magnesium, or preservatives. Storage and Stability This lyophilized protein is stable for six to twelve months when stored desiccated at -20°C to -70°C. After aseptic reconstitution, this protein may be stored at 2°C to 8°C for one month or at -20°C to -70°C in a manual defrost freezer. Avoid Repeated Freeze Thaw Cycles. See Product Insert for exact lot specific storage instructions. Country of Origin USA Shipping Next Day Ambient NCBI Gene Bank Leinco Protein AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Using Recombinant Human Epiregulin (EREG) in research applications is valuable because it is a biologically active growth factor that modulates cell proliferation, differentiation, and signaling, with roles in tissue regeneration, cancer biology, and stem cell research. Key scientific reasons to use recombinant human EREG include:
In summary, recombinant human EREG is a versatile tool for investigating cell signaling, proliferation, differentiation, cancer biology, stem cell function, and tissue regeneration due to its well-characterized bioactivity and relevance in multiple physiological and pathological processes. Recombinant Human Epiregulin can be used as a standard for quantification or calibration in ELISA assays, provided it is compatible with your assay system and matches the native protein’s immunoreactivity. Recombinant standards are commonly used in ELISA kits for quantitative measurement of analytes, including epiregulin. Key considerations:
Best Practices:
Limitations:
In summary, recombinant human epiregulin is suitable as a standard for ELISA quantification, but attention to formulation, compatibility, and validation is essential for accurate calibration. Recombinant Human Epiregulin has been validated primarily for bioactivity assays in published research, with applications focused on its role as a growth factor and signaling molecule in various cellular and tissue models. The following are the main validated applications and experimental contexts:
Summary Table of Validated Applications
Key Points:
No evidence was found for routine use in ELISA, Western blot as a detection reagent, or immunoassays; its primary validation is for functional and bioactivity assays. Reconstitution ProtocolRecombinant Human Epiregulin is supplied as a lyophilized powder and requires proper reconstitution before use in cell culture experiments. The reconstitution process is straightforward but requires attention to specific details to maintain protein activity and stability. Initial Preparation Before reconstituting, briefly centrifuge the vial to concentrate the lyophilized powder at the bottom of the tube. This ensures you are working with all the protein material and prevents loss during the reconstitution process. Remove the plastic cap from the vial and gently tap down any loose powder on the sides to consolidate it. Diluent Selection and Reconstitution Concentration Reconstitute the lyophilized Epiregulin in sterile, high-purity water (18 MΩ-cm H₂O) or sterile phosphate-buffered saline (PBS). The standard reconstitution concentration is 100 μg/mL, though some protocols recommend concentrations between 0.1 to 1.0 mg/mL depending on your experimental requirements. For formulations containing carrier protein, reconstitute in sterile PBS containing at least 0.1% human or bovine serum albumin. Reconstitution Procedure Using a sterile syringe and needle, draw up the calculated volume of diluent needed based on your vial size and desired final concentration. For example, if you have a 750 microgram vial and want a 1 mg/mL solution, draw up 750 microliters of diluent. Insert the needle into the rubber stopper without touching the lyophilized product itself. Dispense the liquid slowly into the vial without forcing it, allowing air to equalize the pressure as needed. Once all liquid is dispensed, gently swirl the vial while avoiding foam formation. Do not vortex the solution, as this can denature the protein. Storage and StabilityLyophilized Protein Storage Store the unopened lyophilized vial at -20 to -70°C for up to 12 months from the date of receipt. Short-term storage at 4°C (up to 6 months) or room temperature (up to 30 days) is permissible if necessary. Reconstituted Protein Storage After reconstitution, store working aliquots at -20 to -80°C for extended storage, with a shelf life of approximately 3 months under these conditions. If stored at 2 to 8°C under sterile conditions, the reconstituted protein remains stable for approximately 1 month. Critically, avoid repeated freeze-thaw cycles, as these can compromise protein integrity and biological activity. Use a manual defrost freezer rather than an automatic defrost model to minimize temperature fluctuations. Biological Activity ConsiderationsThe reconstituted Epiregulin protein is biologically active and will stimulate proliferation in fibroblast cell lines, with an ED₅₀ (effective dose for 50% response) of 0.125-0.75 ng/mL in Balb/3T3 mouse embryonic fibroblasts. The mature secreted form comprises approximately 50 amino residues with a molecular weight of 5.6 kDa. This information is useful for determining appropriate working concentrations for your specific cell culture experiments. References & Citations1. Toyoda, H. et al. (1997) Biochem. J. 326:69 2. Komurasaki, T. et al. (1997) Oncogene 15:2841 3. Shirakata, Y. et al. (2000) J. Biol. Chem. 275:5748 4. Taylor, DS. et al. (1999) Proc. Nat. Acad. Sci. (USA) 96:1633 5. Das, SK. et al. (1997) Dev. Biol. 190:178 Certificate of AnalysisIMPORTANT Use lot specific datasheet for all technical information pertaining to this recombinant protein. |
Related Products
Prod No. | Description |
|---|---|
E322 | |
E314 | |
E152 | |
E317 |
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.