The development of in vitro diagnostics (IVD) kits based on immunoassays, such as lateral flow tests (LFT) and enzyme-linked immunosorbent assay (ELISA) relies on the availability of matched pairs, high-quality antibodies for capture and detection of target analytes. It can be a challenge to find off the shelf and confirmed antibody pairs with the high sensitivity, specificity, purity, and stability needed to ensure an IVD kit meets performance requirements. We have taken the guesswork out of finding confirmed antibodies for diagnostic raw materials offering only the best confirmed pairs.
What are matched pairs?
Matched antibody pairs consist of two antibodies that target non-overlapping regions or epitopes on the same antigen of interest. They can be used in assays such as sandwich ELISA or LFT to detect the antigen by immobilizing one antibody on a substrate to capture the antigen while the second antibody is labeled to detect the captured antigen. In some cases, an antibody may self pair.
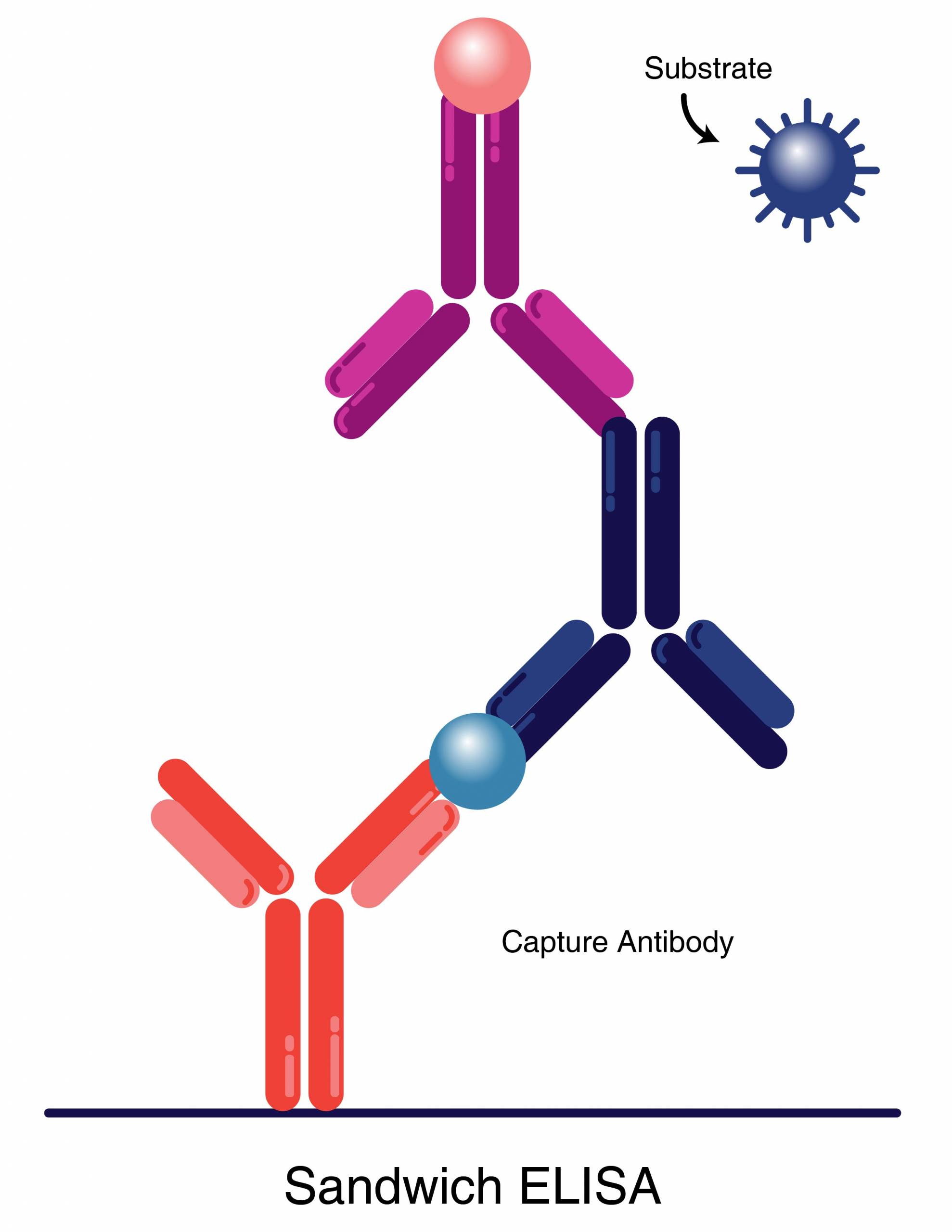
Figure 1. An antigen can be detected by ELISA using a matched pair of antibodies for capture and detection.
How are matched pairs selected for a target antigen?
Matched pairs from Leinco undergo rigorous testing. The antibodies have:
- high affinity and specificity for the target,
- are compatible with each other in terms of their binding sites and reactivity (they do not compete for the same epitope on the target antigen), and
- are labeled to match the application using optimization methods that consider important factors such as stability, signal intensity, and ease of conjugation.
The process for identifying matched pairs can be illustrated by the identification of matched pairs to detect Influenza A, HiN1, strain AWS33 by lateral flow test (link to case study):
- Combinations of antibodies were tested where one antibody is immobilized on nitrocellulose (capture antibody) and the other was conjugated with a gold nanoparticle (detection antibody).
- An aliquot of target pathogen (in this case influenza virus) was added to the nitrocellulose strip.
- The detection antibody was added, and the chromatic change was determined visually (Table 1).
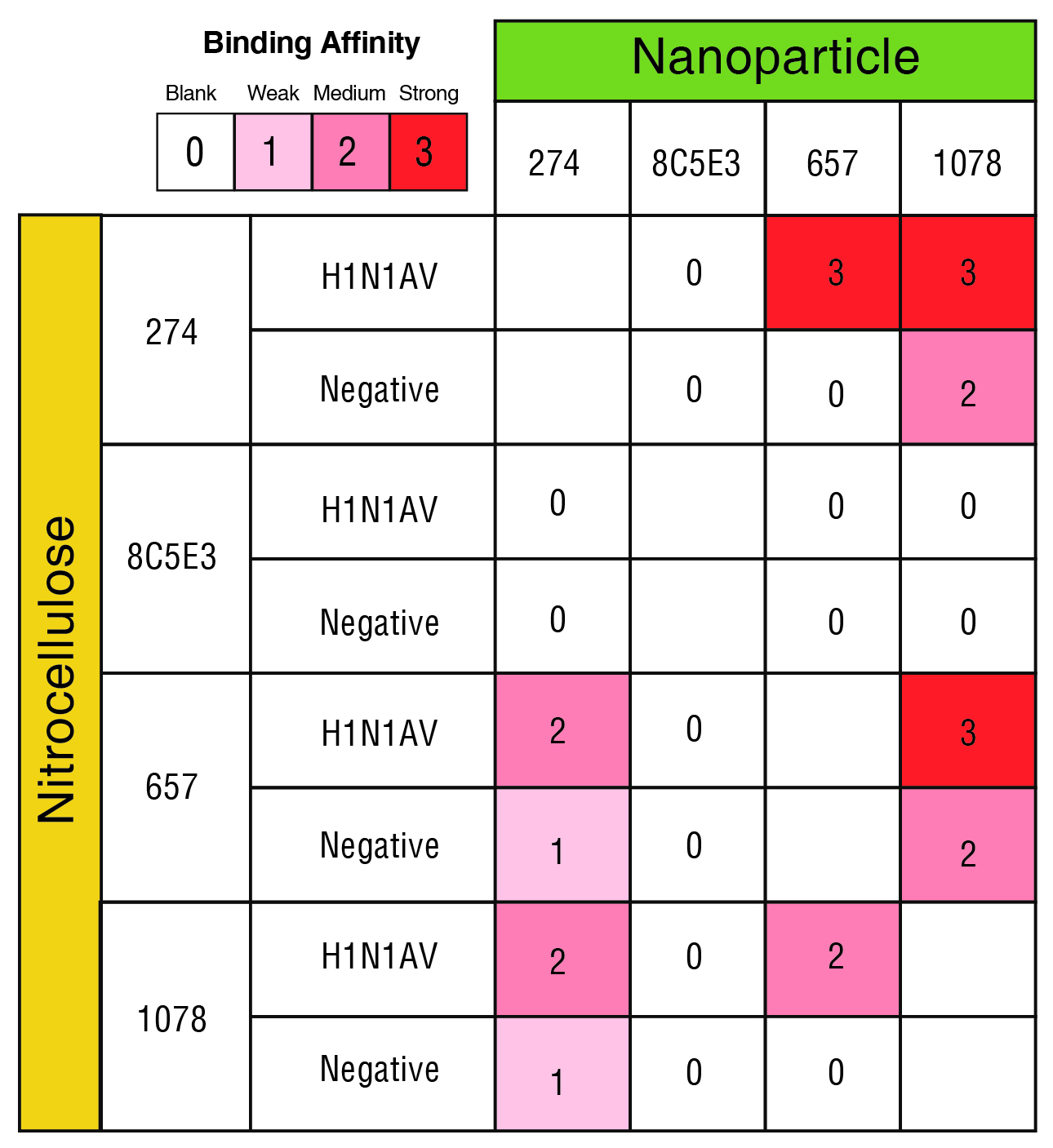
Table 1. Qualitative ability of antibody pairs to detect Influenza A, H1N1, strain AWS33 by lateral flow test
One pairing, antibody 274 for the capture and 657 for the conjugate, showed strong detection with no false positive. Other pairings either had no detectable visual change or gave a false positive and so were discarded from further analysis (Table 1).
The limit of detection (LOD) for the 274/657 matched pair was determined by titrating the virus and measuring pixel intensity using Langmuir Model software image analysis. The performance was compared to commercially available tests, leading to the confirmation of the matched pairs for the application.
Ensuring high kit-specific performance
Once confirmed matched pairs from Leinco have been selected for a specific IVD test, their performance can be further characterized for the intended use:
- Optimization: Optimize the antibody concentrations, sample matrix, and other assay conditions to achieve the desired sensitivity, specificity, and limit of detection. This may involve iterative testing and adjustment of various parameters.
- Cross-reactivity testing: Assess the antibody pairs for potential cross-reactivity with closely related antigens or common interfering substances that may be present in the samples. This helps to ensure specificity and minimize false-positive or false-negative results.
- Stability and storage: Evaluate the stability of the antibodies under relevant storage conditions, including temperature, humidity, and duration. Proper storage and handling are crucial to maintain the functionality of the antibodies during the shelf life of the IVD test.
- Quality control: Implement robust quality control measures throughout the manufacturing process of the lateral flow test to ensure batch-to-batch consistency and reproducibility. This includes regular monitoring of antibody performance, lot-to-lot variation analysis, and appropriate documentation.
If an “off the shelf“ solution isn’t available we are able to test panels of antibodies to find the best pairs for your particular assay.
Readily available validated matched pairs for your application
Leinco can provide “Off the Shelf” validated high quality matched pair antibodies for the development of high-performance diagnostic tests based on ELISA and lateral flow.
As a CDMO, Leinco will also work closely with you throughout every step of the assay development process, assuring our components are optimized for your IVD diagnostic and research needs.
Our range of matched pairs of antibodies focuses on the development of immunological IVD kits for three key areas: Category A pathogens, sexually transmitted diseases (STIs), and nosocomial infections.

