Coxiella Burnetii IgG Phase I ELISA Kit
Data
- -
- -
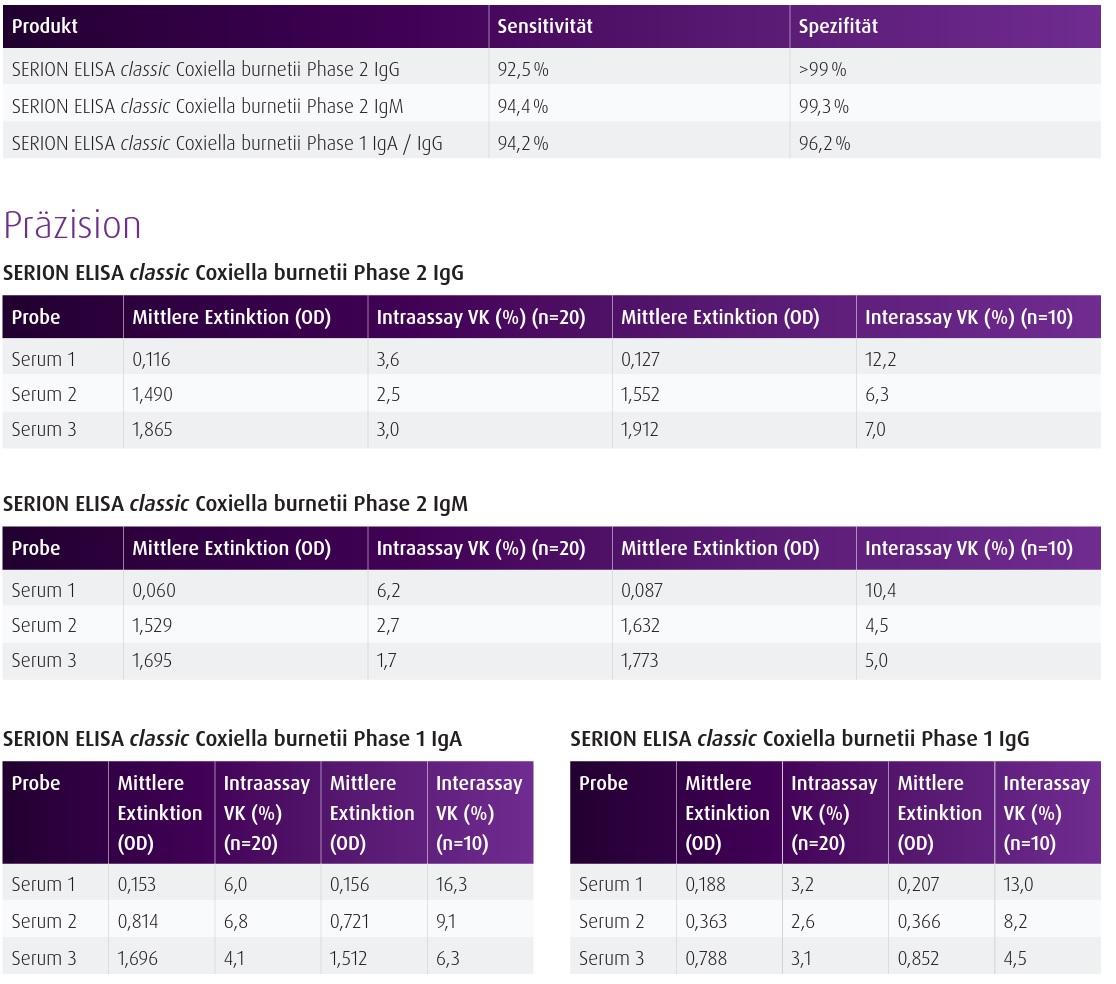
Materials Provided Break apart microtiter test strips each with antigen-coated single wells 8 x 12 (96 Total) Standard serum (ready-to-use) 2 x 2 mL Negative control serum (ready-to-use) 2 mL Anti-human-IgG-conjugate (ready-to-use) 13 mL Washing solution concentrate (sufficient for 1000ml) 33.3 mL Dilution buffer 2 x 50 mL Stopping solution 15 mL Substrate (ready-to-use) 13 mL Quality control certificate with standard curve and evaluation table 1 BackgroundSERION ELISA classic Coxiella burnetii tests are recommended for the detection of human antibodies in serum or plasma directed against Coxiella burnetii in Phase 1 or Phase 2. SERION ELISA classic Coxiella burnetii IgM is recommended for the detection of acute Q-fever, while SERION ELISA classic Coxiella burnetii (Phase 2) IgG supports the differential diagnosis of infections of the respiratory tract, especially atypical pneumonia. SERION ELISA classic Coxiella burnetii (Phase I) tests are recommended for the diagnosis of chronic Q-fever. All SERION ELISA classic Coxiella burnetii are used for the serological therapy follow-up in acute and chronic diseases. SERION ELISA classic Coxiella burnetii Phase 1 IgG/IgA resp. Product is intended for research use. Additional Serion Kit Information For Sale In the United States (USA) Only. For International Inquires Please Contact Virion-Serion |