Measles Virus IgM ELISA Kit
Data
- -
- -
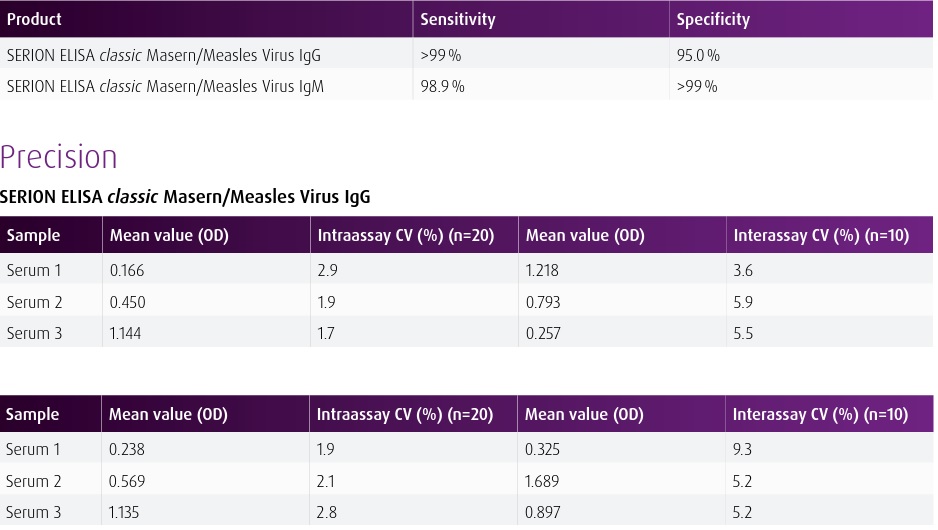
Materials Provided Break apart microtiter test strips each with antigen coated single wells 8 x 12 (96 Total) Standard serum (ready-to-use) 2 x 2 mL Negative control serum (ready-to-use) 2 mL Anti-human-IgG-conjugate (ready-to-use) 13 mL Washing solution concentrate (sufficient for 1000ml) 33.3 mL Dilution buffer 2 x 50 mL Stopping solution 15 mL Substrate (ready-to-use) 13 mL Quality control certificate with standard curve and evaluation table 1 BackgroundThe SERION ELISA classic Masern/Measles Virus IgG and IgM tests are quantitative and qualitative immunoassays for the detection of human antibodies in serum or plasma directed against Measles Virus. The SERION ELISA classic Masern/Measles Virus IgM test is recommended for the determination of acute infections. The SERION ELISA classic Masern/Measles Virus IgG assay is recommended for determination of immune status and for detection of intrathecal synthesized IgG antibodies in cerebrospinal fluid for CSF diagnostics. SERION ELISA classic Measles Virus IgG/IgM are quantitative and qualitative tests for detection of human antibodies in serum, plasma or cerebrospinal fluid against Measles Virus. Product is intended for research use. NOTE: Pretreatment of samples with RF-Absorbent (Z200) is recommended for use with IgM ELISA kits to eliminate presence of sample rheumatoid factors and possible false negative results. Additional Serion Kit Information For Sale In the United States (USA) Only. For International Inquires Please Contact Virion-Serion |