Mikrogen Diagnostik recomLine HEV IgG/IgM Lateral Strip Test Kit
Data
- -
- -
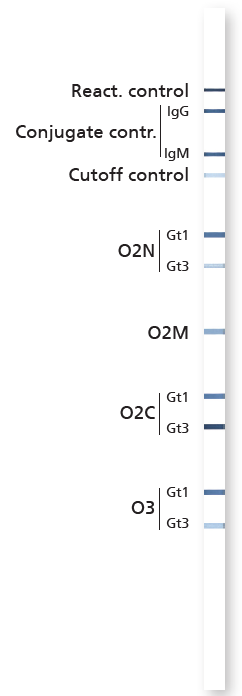
Product DetailsDescription Hepatitis E virus (HEV) is one of the leading causes of faecal-orally acquired hepatitis worldwide, usually resulting from contaminated drinking water. In developed countries, hepatitis E is usually transmitted through infected pork, which was not sufficiently cooked. HEV can be transmitted through blood products, blood transfusions and organ donation. In Europe most cases of hepatitis E are caused by the HEV genotype 3. The HEV infection usually remains asymptomatic. Acute hepatitis E is an illness with a clinical presentation comparable to hepatitis A. Typical signs of hepatitis E include flu-like symptoms, vomiting, diarrhoea, fever, arthralgia and headache usually associated with a rise in liver enzyme values. Cholestatic jaundice that develops during the course of disease can persist for several weeks. HEV infection is usually self-limiting. In endemic regions in a high percentage of cases HEV infections during pregnancy follow a fulminant course, accompanied by a high mortality rate of approx. 20%. In males and non-pregnant females the mortality rate is 0.5% - 4.0%. The recomLine HEV IgG/IgM uses purified recombinant antigens, thus reproducibly ensuring high sensitivity and specificity. The selection of homologous proteins from two different genotypes (genotype 1 and genotype 3) facilitates a high sensitivity also in non-endemic areas with a high percentage of autochthonous cases of hepatitis E. Research Use Only. For Sale In the United States Only. To purchase outside of the US, please contact Mikrogen. Country of Origin Germany Related ProtocolsLateral Flow |