Parainfluenza Virus IgA ELISA Kit
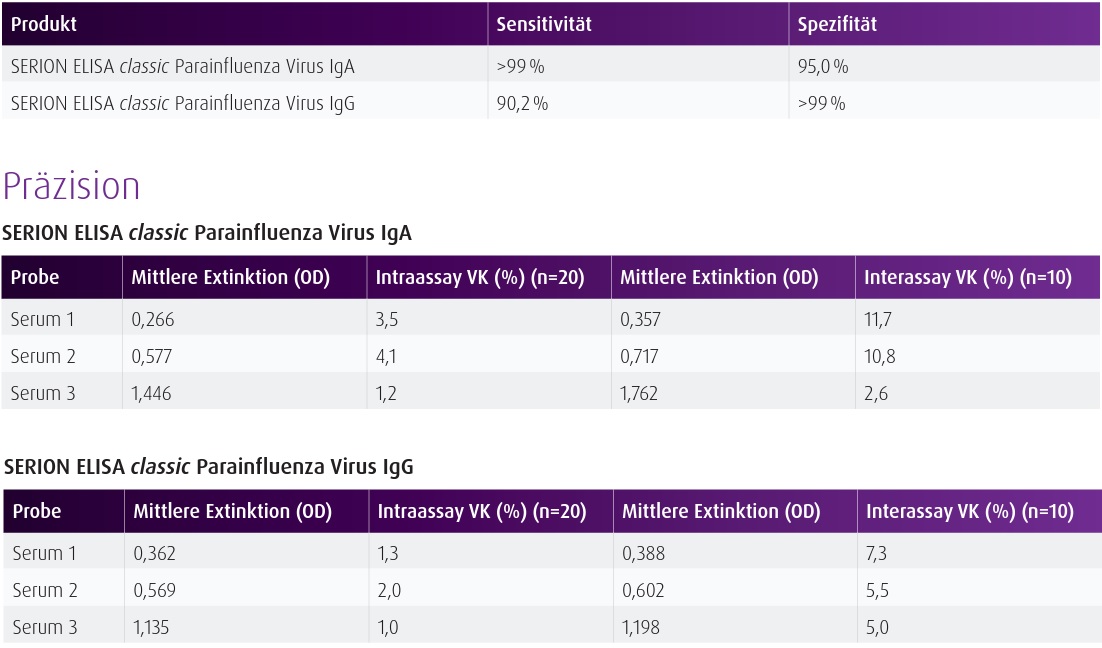
Data
- -
- -
Materials Provided Break apart microtiter test strips each with antigen-coated single wells 8 x 12 (96 Total) Standard serum (ready-to-use) 2 x 2 mL Negative control serum (ready-to-use) 2 mL Anti-human-IgA-conjugate (ready-to-use) 13 mL Washing solution concentrate (sufficient for 1000ml) 33.3 mL Dilution buffer 2 x 50 mL Stopping solution 15 mL Substrate (ready-to-use) 13 mL Quality control certificate with standard curve and evaluation table 1 BackgroundThe SERION ELISA classic Parainfluenza Virus IgA and IgG tests are qualitative and quantitative immunoassays for the detection of human antibodies in serum or plasma directed against all relevant human pathogenic Parainfluenza Viruses. They allow for the detection of acute infections as well as for confirmation of contact with the pathogen in the differential diagnosis of respiratory infections. The SERION ELISA classic Parainfluenza Virus IgA test is a qualitative and quantitative immunoassay for the detection of human antibodies in serum or plasma directed against all relevant human pathogenic Parainfluenza Viruses. Product is intended for research use. Additional Serion Kit Information For Sale In the United States (USA) Only. For International Inquires Please Contact Virion-Serion |