Recombinant Human Brain-derived neurotropic factor (BDNF)
Data
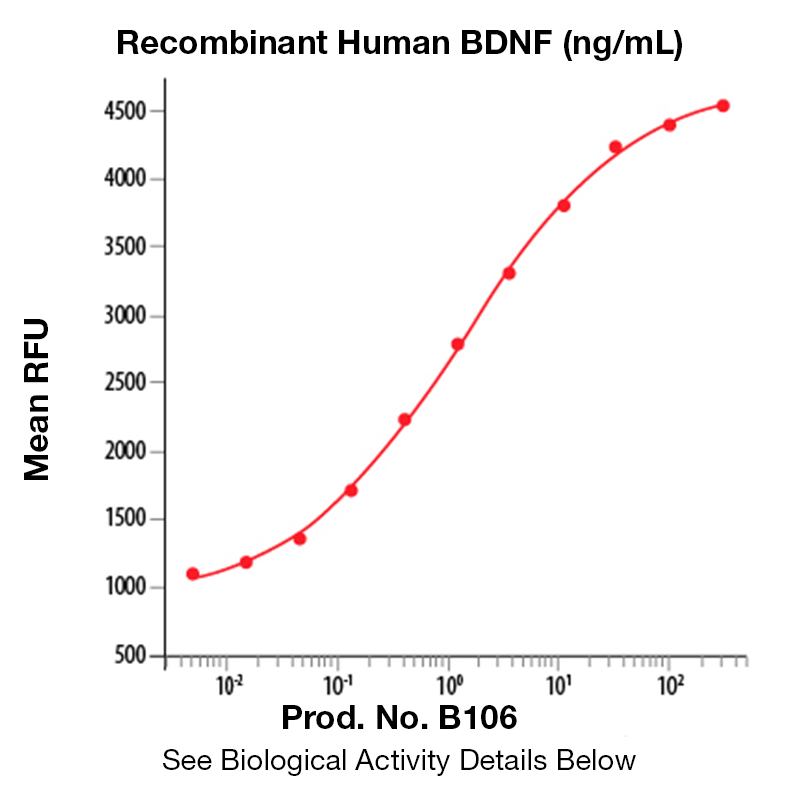
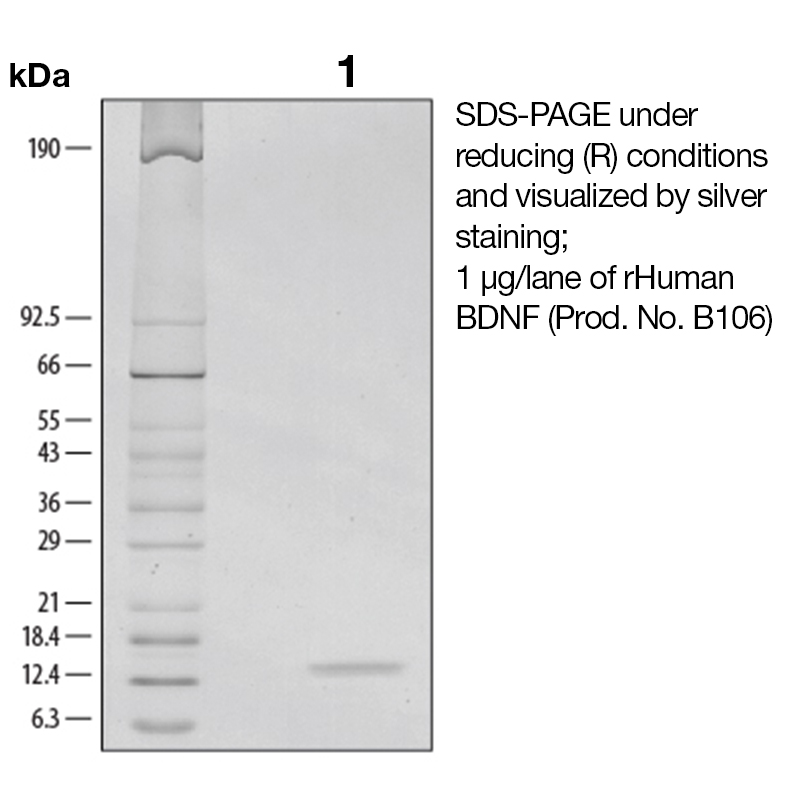
BackgroundBrain-derived neurotropic factor (BDNF), also known as MGC34632, is a member of the NGF family of neurotrophic growth factors that includes NGF, NT-3, and NT-4/5. Like other members of this family, BDNF supports neuron proliferation and survival (1). It acts on certain neurons of the central and peripheral nervous systems, helping to support the survival of existing neurons and encourage the growth and differentiation of new neurons and synapses. In the brain, BDNF is active in the hippocampus, cortex, and basal forebrain, areas vital to learning, memory and higher thinking (2). Despite its name, BDNF is actually found in a range of tissue and cell types, not just in the brain. It is also expressed in the retina, the CNS, motor neurons, the kidneys and the prostate. BDNF binds at least two receptors on the surface of cells which are capable of responding to this growth factor, TrkB and the LNGFR (3). Expression of BDNF is reduced in both Alzheimer's and Huntington disease patients (4-5). In addition, functional studies showed that age-associated changes in BDNF-mediated pathways can enhance inflammation and increase myocardial injury after myocardial infarction in the aging heart (6). Various studies have shown possible links between BDNF and other conditions, such as depression, schizophrenia, obsessive-compulsive disorder, Rett syndrome, dementia, anorexia nervosa, bulimia nervosa, epilepsy and eczema. Protein DetailsPurity >97% by SDS-PAGE and analyzed by silver stain. Endotoxin Level <1.0 EU/µg as determined by the LAL method Biological Activity The biological activity of Human BDNF was determined by its ability to stimulate proliferation of the TrkB transfected cell line, Baf-TrkB. The expected ED<sub>50</sub> for this effect is typically 3 - 10 ng/ml. Amino Acid Sequence hsdparrgel svcdsisewv taadkktavd msggtvtvle kvpvskgqlk qyfyetkcnp mgytkegcrg idkrhwnsqc rttqsyvral tmdskkrigw rfiridtscv ctltikrgr N-terminal Sequence Analysis His129 State of Matter Lyophilized Predicted Molecular Mass The predicted molecular weight of Recombinant Human BDNF is Mr 13.5 kDa However, the actual molecular weight as observed by migration on SDS-PAGE is Mr 13-14 kDa (reducing conditions) and 11-12 kDa (non-reducing conditions). Predicted Molecular Mass 13.5 Formulation This recombinant protein was 0.2 µm filtered and lyophilized from a filtered solution in 100mM Sodium Citrate and 300 mM NaCl, pH 3.0. Storage and Stability This lyophilized protein is stable for six to twelve months when stored desiccated at -20°C to -70°C. After aseptic reconstitution, this protein may be stored at 2°C to 8°C for one month or at -20°C to -70°C in a manual defrost freezer. Avoid Repeated Freeze Thaw Cycles. See Product Insert for exact lot specific storage instructions. Country of Origin USA Shipping Next Day Ambient NCBI Gene Bank Leinco Protein AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Recombinant human BDNF is a valuable tool for neuroscience research due to its well-characterized biological functions and therapeutic potential across multiple experimental applications. Neuronal Support and SurvivalRecombinant BDNF provides robust support for neuronal populations in both central and peripheral nervous systems. The protein selectively promotes survival and differentiation of specific neuronal subpopulations, including sensory neurons, sympathetic neurons, and dopaminergic midbrain neurons. This selectivity makes it particularly useful for studies focusing on specific neuronal populations without affecting non-target cells. Synaptic Function and PlasticityBDNF exerts potent effects on synaptic architecture and function. The protein promotes axon elongation and branching in sensory neurons, while also enhancing dendritic outgrowth in neuronal cultures. At the synaptic level, BDNF increases the number of docked synaptic vesicles and potentiates synaptic efficiency, making it ideal for investigating synaptic plasticity mechanisms. These properties are particularly valuable for studying learning and memory processes, as BDNF expression in the hippocampus is essential for long-term memory storage. Cognitive and Behavioral ApplicationsRecombinant BDNF improves cognitive function by enhancing neuronal plasticity and increasing acetylcholinesterase activity. The protein has demonstrated efficacy in promoting axonal regeneration, maintaining synaptic strength, and preventing neuronal loss in various neurodegenerative disease models. Additionally, BDNF supports the development and regeneration of serotonergic and dopaminergic neurons, enabling investigation of neurotransmitter systems. Disease Modeling and Therapeutic DevelopmentBDNF is particularly relevant for research on neurodegenerative diseases, as reduced BDNF expression is associated with Alzheimer's disease and Huntington's disease pathology. Using recombinant BDNF allows you to model neuroprotective mechanisms and evaluate potential therapeutic interventions. The protein's ability to reduce amyloid-beta toxicity and enhance learning and memory capability makes it suitable for Alzheimer's disease research. Experimental FlexibilityRecombinant BDNF is available in multiple formulations, including carrier-free preparations, allowing you to customize your experimental design based on specific requirements. The protein's bioactivity can be measured through standardized assays, such as proliferation induction in established cell lines, ensuring reproducibility across experiments. Yes, recombinant human BDNF can be used as a standard for quantification or calibration in ELISA assays, provided it is well-characterized and matches the isoform detected by your assay. Recombinant BDNF is commonly employed as a calibrator in commercial ELISA kits for quantitative measurement of BDNF in biological samples. Key considerations for use as a standard:
Protocol best practices:
Limitations and caveats:
In summary, recombinant human BDNF is suitable as a standard for ELISA calibration, provided it is matched to the assay’s specificity and validated for use in your protocol. Recombinant human brain-derived neurotrophic factor (BDNF) has been validated in published research for a range of applications, primarily in neuroprotection, neuroregeneration, and as a therapeutic candidate in neurological and neurodegenerative disease models. Key validated applications include:
Experimental techniques and protocols where recombinant BDNF is validated:
Summary Table: Validated Applications of Recombinant Human BDNF
In summary, recombinant human BDNF is a validated tool in both basic and translational neuroscience research, with applications spanning from in vitro neuronal assays to in vivo models and clinical trials for neurodegenerative and neurological disorders. To reconstitute and prepare Recombinant Human Brain-derived Neurotrophic Factor (BDNF) for cell culture experiments, dissolve the lyophilized protein in sterile water or buffer to a concentration between 0.1–1.0 mg/mL, then dilute to your working concentration in cell culture medium or buffer containing a carrier protein such as BSA or HSA to minimize adsorption and maintain stability. Detailed protocol and best practices:
Summary Table: Key Steps for BDNF Reconstitution
These steps ensure maximal stability and bioactivity of recombinant BDNF for cell culture experiments. Always consult the specific product’s Certificate of Analysis for any additional manufacturer recommendations. References & Citations1. Acheson, A. et al. (1995) Nature 374:450 2. Yamada, K. et al. (2003) J. Pharmacol. Sci. 91 :267 3. Patapoutian, A. et al. (2001) Curr. Opin. Neurobiol. 11:272 4. Zuccato, C. et al. (2009) Nat. Rev. Neurol. 5:311 5. Zajac, MS. et al. (2009) Hippocampus 20:621 6. Cai, D. et al. (2006) Physiol. Genomics 24:191 Certificate of AnalysisIMPORTANT Use lot specific datasheet for all technical information pertaining to this recombinant protein. |
Related Products
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.