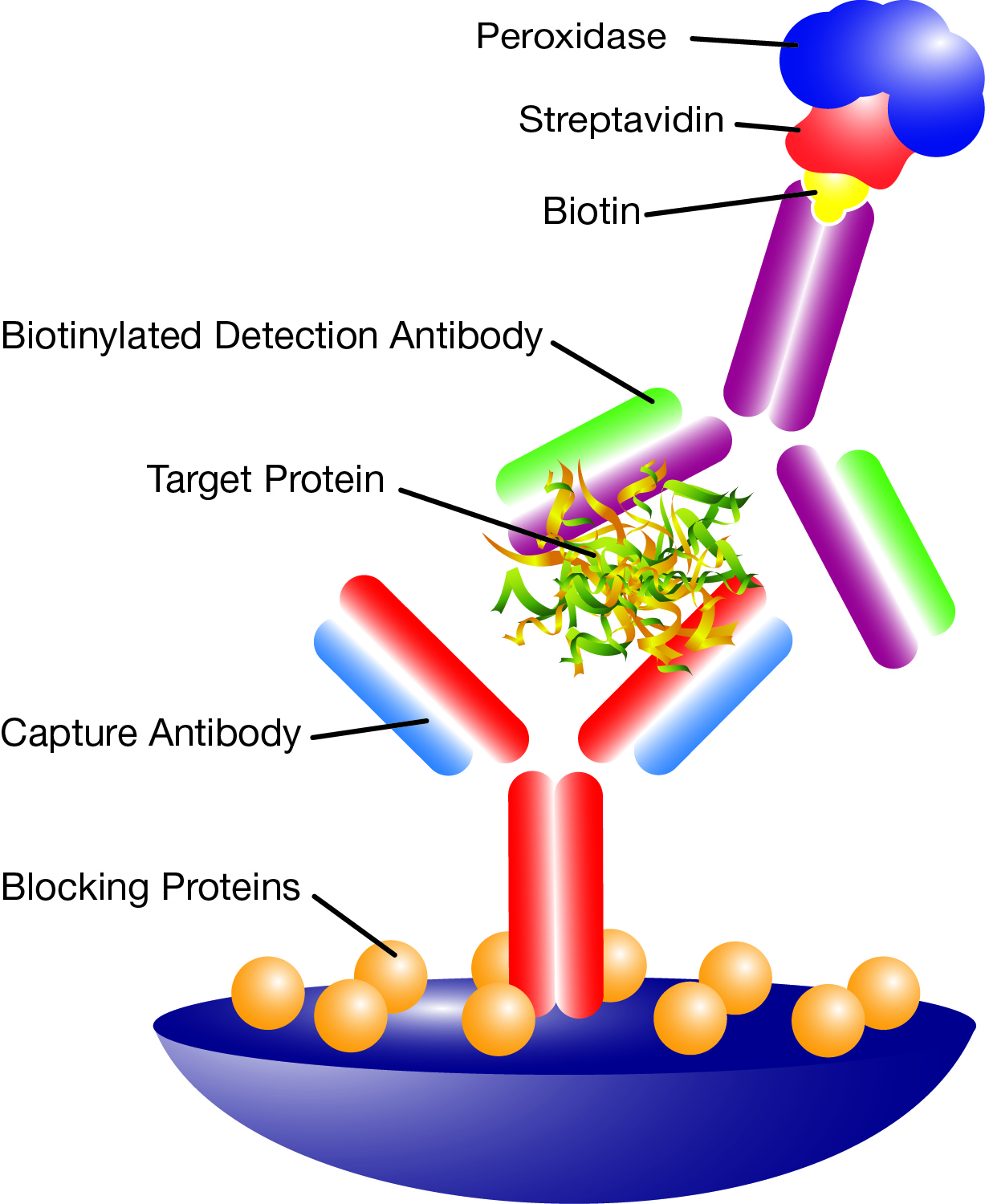
Biotin, Avidin, and Streptavidin
Streptavidin is a tetrameric bacterial protein isolated from the bacterium Streptomyces avidinii. Like avidin from egg whites, streptavidin can bind four molecules of biotin and has one of the highest noncovalent affinity constants known in nature (Kd ~ 10-15). This protein is composed of four identical subunits with a molecular weight of approximately 53,000 daltons. They are also free of carbohydrate side chains and has a near neutral isoelectric point of 6.5.
Streptavidin has a wide variety of applications such as use as a second step reagent with biotin labeled primary antibodies or in the formation of class I and II MHC tetramers for detection of antigen specific T-cells. Leinco Technologies offers streptavidin conjugated to a wide spectrum of fluorescent dyes and enzymes for use as a second step reagent for detection of biotin labeled primary antibodies and other biotin labeled molecules. The advantage of this protein is the amplification of signal achieved due to binding of multiple streptavidin conjugated complexes to one biotin labeled primary antibody. Or in the case of a tetramer, the binding of multiple biotinylated immune system molecules to one streptavidin to increase detection sensitivity of antigen specific T-cells.
UltraAvidin™
UltraAvidin™ is Leinco Technologies’ trademark for a uniquely modified form of avidin which is isolated from chicken egg whites. With a molecular weight of 60,000 there are four identical subunits each capable of binding one molecule of biotin. UltraAvidin™ from Leinco Technologies has been deglycosylated to prevent carbohydrate moieties from adhering to lectin-like receptors on the surface of cells, thus eliminating the possibility of false positives. Unlike native avidin, UltraAvidin™ has a near neutral pI which prevents electrostatic interactions with negatively charged serum or membrane proteins.
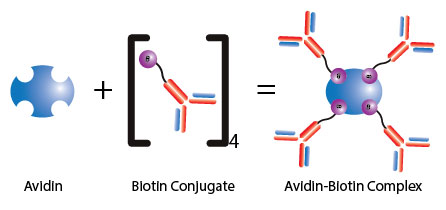
Above: Diagram of signal amplification by avidin-biotin complex formation with an antibody. UltraAvidin or streptavidin Protein can bind four (4) moles of biotin per mole of protein, and it shows extremely high affinity normally conjugated to an enzyme, antibody or target protein to form an avidin-biotin complex.
Conjugates
Leinco’s Streptavidin and UltraAvidin™ products are available in a variety of conjugation formats: Purified, PE, PerCP, FITC, HRPO, APC, Alk Phos, and Dylight® 405, 488, 550, 594, 650.
Applications for which the avidin-biotin interaction is used include:
- ELISA
- Tetramer Staining
- Immunohistochemistry (IHC)
- Immunofluorescence Microscopy
- Western Blotting
- Immunoprecipitation (IP)
- Signal Amplification using Steptavidin
- Microarrays
- Flow Cytometry (FACS)
- in situ hybridization