High Functional Grade Antibodies: SARS CoV-2 and Influenza Variants
The Omicron variant of SARS-CoV-2, the virus that causes COVID-19, was first detected in November, 2021 in Botswana and South Africa with the first confirmed case of the variant identified in California in December, 2021 and within 3 weeks was present in 87 countries worldwide. Since its discovery, Omicron has been associated with a rapid resurgence of infections and has been designated a variant of concern by the World Health Organization. Omicron is exceptional because it carries over 30 mutations to the spike protein, leading to an increased risk of reinfection to those either vaccinated or with natural immunity2. The variant has also been proven to be highly transmissible and has surpassed the Delta variant in many regions of the world.
Recently, the first case of someone infected with the novel coronavirus and influenza was detected in Israel3. The patient was an unvaccinated pregnant woman who was experiencing mild symptoms, but this has led experts to call the combination of viruses “flurona3” and fear that it could become the next issue weighing heavily on the medical system. After a nearly flu-free winter in 2020, researchers are working diligently to try to combat the increasing number of Influenza cases. While the risk of co-infection4 remains relatively low, more research is needed to better understand the interactions between the two viruses and to see if the severity of illness is higher with co-infection.
Response to Ongoing Viral Threats
Leinco Technologies continues to manufacture key bioreagents such as recombinant proteins, antibodies and detection kits for use in COVID-19 and other infectious disease research applications. Our extensive line of SARS-CoV- 2 and Influenza antibodies and antigens are produced at one of the highest functional purity levels in the industry. Partnering with Leinco allows you to scale-up your current production of antibodies and reagents, allowing you to respond to novel threats more effectively and efficiently. The goal of our collaborations are to shorten the time between a new virus discovery and the development of diagnostic or research tools made available to the scientific community worldwide. After 30 years in the life science tools industry, our goal remains the same to enable reproducible science: provide the highest purity antibodies and proteins possible and make them widely available to life science researchers and developers of diagnostic test kits.
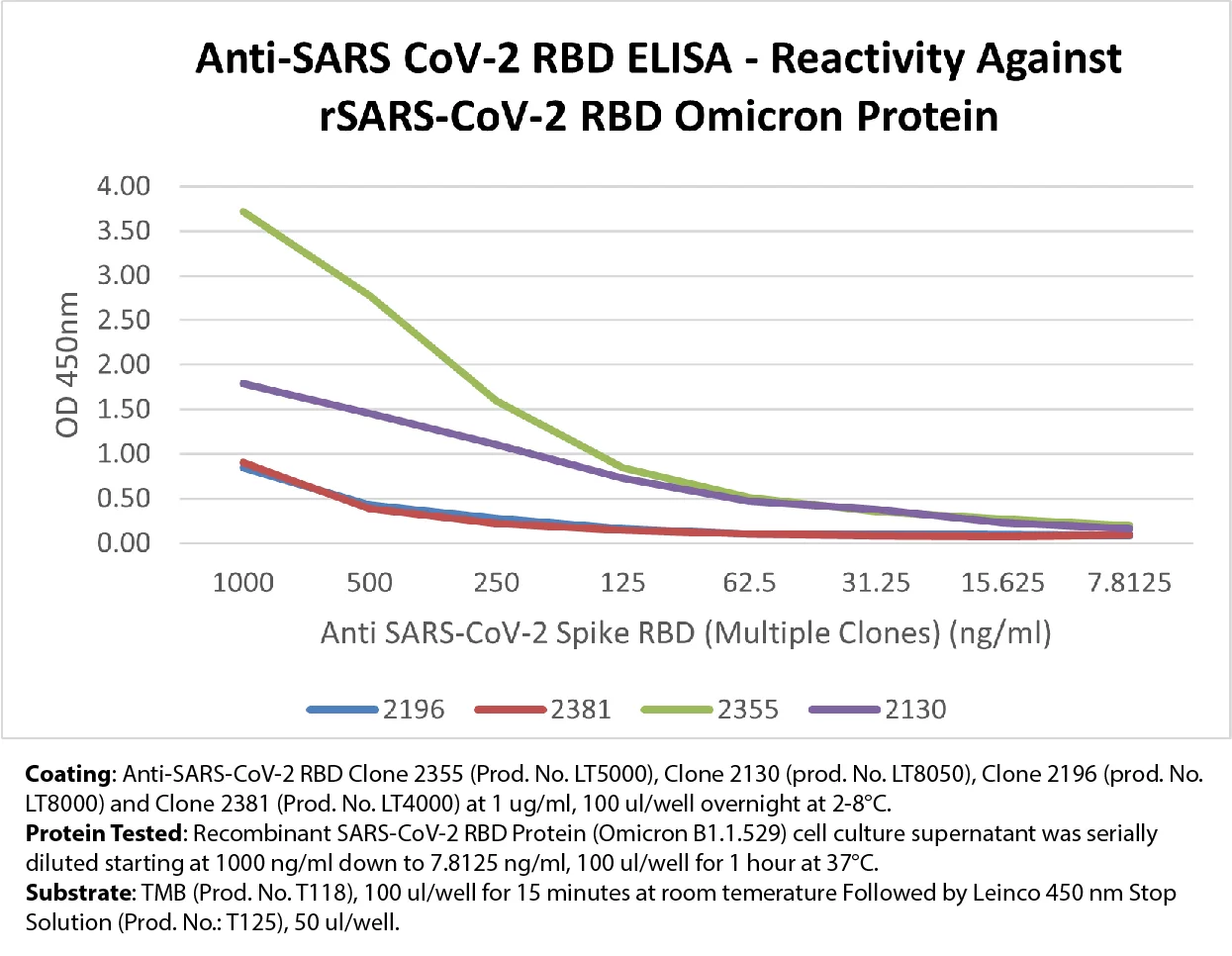
SARS-CoV-2 COVID-19 Omicron
The Omicron variant was designated as a Variant of Concern (VOC) by The World Health Organization (WHO). In response to this, Leinco Technologies has developed Omicron Spike RBD protein and antibodies reacting with Omicron to support the research of the Omicron variant. The structural proteins of SARS-CoV-2 spike (S), membrane (M), envelope (E), and nucleocapsid (N) are cytological markers for viral sites of infection including cell-to-cell transmissibility. View our Antibody and Recombinant Protein products focused on SARS-CoV-2 COVID-19 Omicron listed below.
Product | Clone | Applications | Prod No. | Package Size |
|---|---|---|---|---|
2130 | ELISA | LT8050 | 100 µg ⋅ 500 µg | |
2196 | ELISA | LT8000 | 100 µg ⋅ 500 µg | |
2355 | ELISA ⋅ N | LT5000 | 100 µg ⋅ 500 µg | |
2381 | ELISA | LT4000 | 100 µg ⋅ 500 µg |
Influenza A & B
Human influenza A and B viruses cause seasonal epidemics of disease (known as flu season) almost every winter in the United States. The influenza A virus nucleocapsid or nucleoprotein (NP) is an essential multifunctional protein that encapsidates the viral genome and plays a critical role in virus replication and host adaptation. These are key in developing specific In Vitro Diagnostic (IVD) testing. View our recombinant Influenza nucleocapsid products from Leinco that can aid in your influenza research below.
Product | Clone | Applications | Prod No. | Package Size |
|---|---|---|---|---|
ELISA ⋅ WB | F600 | 100 µg ⋅ 500 µg | ||
ELISA ⋅ WB | F650 | 100 µg ⋅ 500 µg |