Anti-Human EGFR (Cetuximab) [Clone C225]
Anti-Human EGFR (Cetuximab) [Clone C225]
Product No.: LT600
Product No.LT600 Clone C225 Target EGFR Product Type Biosimilar Recombinant Human Monoclonal Antibody Alternate Names ErbB-1; HER1; epidermal growth factor receptor Isotype Human IgG1κ Applications CyTOF® , ELISA , FA , FC , ICC , IHC |
Data
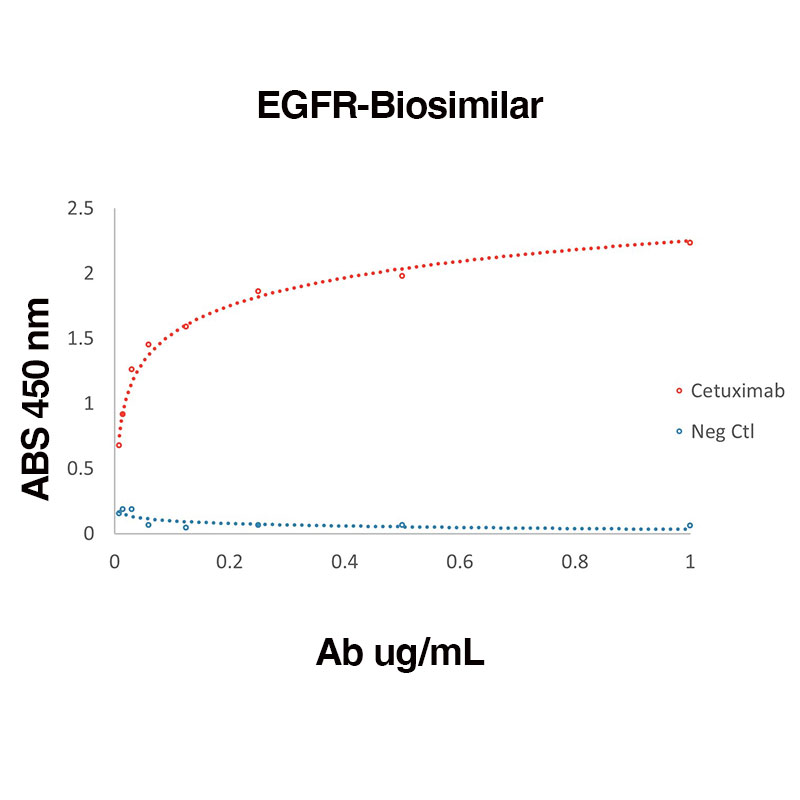 Direct binding of Human Recombinant EGFR (Leinco Prod. No.: E309) to anti-Human EGFR Cetuximab (Leinco Prod. No.: LT600)
Direct binding of Human Recombinant EGFR (Leinco Prod. No.: E309) to anti-Human EGFR Cetuximab (Leinco Prod. No.: LT600)
Binding was measured by ELISA. Recombinant Human EGFR was immobilized at 1 µg/mL. Cetuximab antibody was titrated.
Antibody DetailsProduct DetailsReactive Species Human Host Species Human Expression Host HEK-293 Cells FC Effector Activity Active Immunogen Human EGFR/ErbB1 Product Concentration ≥ 5.0 mg/ml Endotoxin Level < 1.0 EU/mg as determined by the LAL method Purity ≥95% by SDS Page ⋅ ≥95% monomer by analytical SEC Formulation This biosimilar antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. State of Matter Liquid Product Preparation Recombinant biosimilar antibodies are manufactured in an animal free facility using only in vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Pathogen Testing To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s recombinant biosimilar antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Regulatory Status Research Use Only (RUO). Non-Therapeutic. Country of Origin USA Shipping 2-8°C Wet Ice RRIDAB_2893971 Applications and Recommended Usage? Quality Tested by Leinco FC The suggested concentration for Cetuximab biosimilar antibody for staining cells in flow cytometry is ≤ 0.25 μg per 106 cells in a volume of 100 μl. Titration of the reagent is recommended for optimal performance for each application. Additional Applications Reported In Literature ? FA, ELISA, CyTOF® Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity This non-therapeutic biosimilar antibody uses the same variable region sequence as the therapeutic antibody Cetuximab. This product is for research use only. Cetuximab activity is directed against Human EGFR. Background EGFR is a 170 kD transmembrane glycoprotein that is part of the ErbB family of receptors within the protein kinase superfamily. EGFR is one of four closely related receptor tyrosine kinases: EGFR (ErbB-1), HER2/c-neu (ErbB-2), Her 3 (ErbB-3) and Her 4 (ErbB-4). EGFR is essential for various processes including controlling cell growth and differentiation and ductal development of the mammary glands. Ligand binding induces dimerization and autophosphorylation. It consists of a glycosylated extracellular domain which binds to EGF and an intracellular domain with tyrosine-kinase activity necessary for signal transduction. TGFα, vaccinia virus growth factor, and related growth factors can also bind to and signal through EGFR. Abnormal EGFR signaling has been implicated in inflammatory diseases such as psoriasis, eczema and atherosclerosis. Alzheimer's disease is linked with poor signaling of the EGFR and other receptor tyrosine kinases. Furthermore, over-expression of the EGFR is linked with the growth of various tumors. EGFR has been identified as an oncogene, a gene which in certain circumstances can transform a cell into a tumor cell, which has led to the therapeutic development of anticancer EGFR inhibitors. EGFR is a well-established target for both mAbs and specific tyrosine kinase inhibitors. Anti-Human EGFR (Cetuximab) utilizes the same variable regions from the therapeutic antibody Cetuximab making it ideal for research projects. Antigen Distribution EGFR is ubiquitously expressed and found in the plasma membrane. PubMed NCBI Gene Bank ID UniProt.org Research Area Biosimilars Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Research-grade Cetuximab biosimilars are commonly used as calibration standards or reference controls in pharmacokinetic (PK) bridging ELISAs to create a standard curve for quantifying drug concentrations in serum samples. These biosimilar standards are carefully calibrated against commercial reference Cetuximab (Erbitux™) to ensure accuracy and comparability across both biosimilar and reference products. Key steps and rationale:
Supporting details:
In summary, research-grade Cetuximab biosimilars serve as validated calibration standards in PK bridging ELISAs after establishing their equivalence to the reference (originator) product, supporting accurate measurement of drug concentrations in serum samples for pharmacokinetic and biosimilarity analyses. When studying tumor growth inhibition and characterizing tumor-infiltrating lymphocytes (TILs) using a research-grade anti-EGFR antibody, researchers often use both syngeneic and humanized mouse models. Here are the primary models in which these studies are conducted: Syngeneic ModelsSyngeneic mouse models are widely used for preclinical evaluation of cancer therapies because they allow the study of tumor development and immune interactions in an immunocompetent environment. While syngeneic models typically involve murine tumor cells, they can be engineered to express human antigens, making them suitable for studying human-specific targets like EGFR. However, the use of syngeneic models for studying anti-EGFR antibodies specifically might be limited due to the murine origin of the tumors, which may not fully replicate human EGFR biology. Humanized ModelsHumanized mouse models, particularly those with human immune systems, are ideal for studying the effects of anti-EGFR antibodies on tumor growth and TILs. These models can be engineered to express human EGFR, allowing for more accurate representation of human tumor biology. Athymic (nude) mice are also used, as they can be implanted with human tumor cells (e.g., A431 cells) to study the effects of anti-EGFR therapies like Nanobodies. Humanized models provide a more relevant context for studying the immune-tumor microenvironment, including TILs, when using anti-EGFR antibodies. In terms of specific models, A431-derived solid tumors in athymic mice are a common choice for studying anti-EGFR therapies, as demonstrated with the use of Nanobodies. These models allow researchers to assess the efficacy of anti-EGFR treatments on human tumors in a living organism, providing insights into how these therapies might impact TILs and overall tumor growth. When selecting between syngeneic and humanized models, researchers consider factors like the need for an immunocompetent environment, the specific antigen expression, and the relevance to human tumor biology. For studying anti-EGFR antibodies and TILs, humanized models are generally preferred due to their ability to more accurately reflect the human immune-tumor interaction. Researchers use Cetuximab biosimilars in combination with immune checkpoint inhibitors (such as anti-CTLA-4 or anti-LAG-3 biosimilars) to study synergistic effects in complex immune-oncology models by leveraging Cetuximab’s immunomodulatory properties alongside the inhibitory mechanisms of checkpoint blockade. This strategy is based on the complementary actions of Cetuximab (an EGFR-targeting antibody) and ICIs to enhance both innate and adaptive anti-tumor immune responses. Key mechanisms in preclinical and translational models:
Synergy Rationale:The combination of Cetuximab biosimilars with checkpoint inhibitors often results in:
Experimental Approaches:
Research Tools:
There is limited direct data on specific use with biosimilar anti-CTLA-4 or biosimilar anti-LAG-3, but the mechanistic rationale and protocols are consistent across anti-CTLA-4, anti-PD-1, and anti-LAG-3 inhibitors, especially in complex immune-oncology models. In summary, Cetuximab biosimilars combined with checkpoint inhibitors are studied for their synergistic effects by leveraging distinct immune pathways: Cetuximab recruits and primes immune cells, while ICIs unleash their activity by blocking inhibitory signals, with synergy tested in complex tumor models using immune cell profiling and response rates as endpoints. In a bridging anti-drug antibody (ADA) ELISA for immunogenicity testing of cetuximab biosimilars, the biosimilar itself is used as both the capture and detection reagent to specifically detect patient antibodies directed against the therapeutic drug. Assay Principle and Role of the Cetuximab Biosimilar:
Why a Biosimilar Is Used:
Summary Table: Key Steps in a Bridging ADA ELISA Using a Cetuximab Biosimilar
Important Considerations:
Literature Examples:
This approach is foundational in biotherapeutic immunogenicity testing and enables robust detection of patient immune responses to cetuximab or its biosimilars. References & Citations1. Tortora, G. et al. (1999) Clin Cancer Res. 5(4):909-16. 2. Myers, J. et al. (2006) Clin Cancer Res. 12(2): 600–607. Technical ProtocolsCertificate of Analysis |
Formats Available
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.


