mAbMods™ Anti-Mouse TREM2 [Clone 178 (LALAPG)] — Fc Muted™
mAbMods™ Anti-Mouse TREM2 [Clone 178 (LALAPG)] — Fc Muted™
Product No.: T721
Product No.T721 Clone 178 (LALAPG) Product Type Recombinant Monoclonal Antibody for in vivo Use Alternate Names Triggering receptor expressed on myeloid cells 2 Isotype Mouse IgG2a Applications B , ELISA , FA , FC , in vivo |
Data
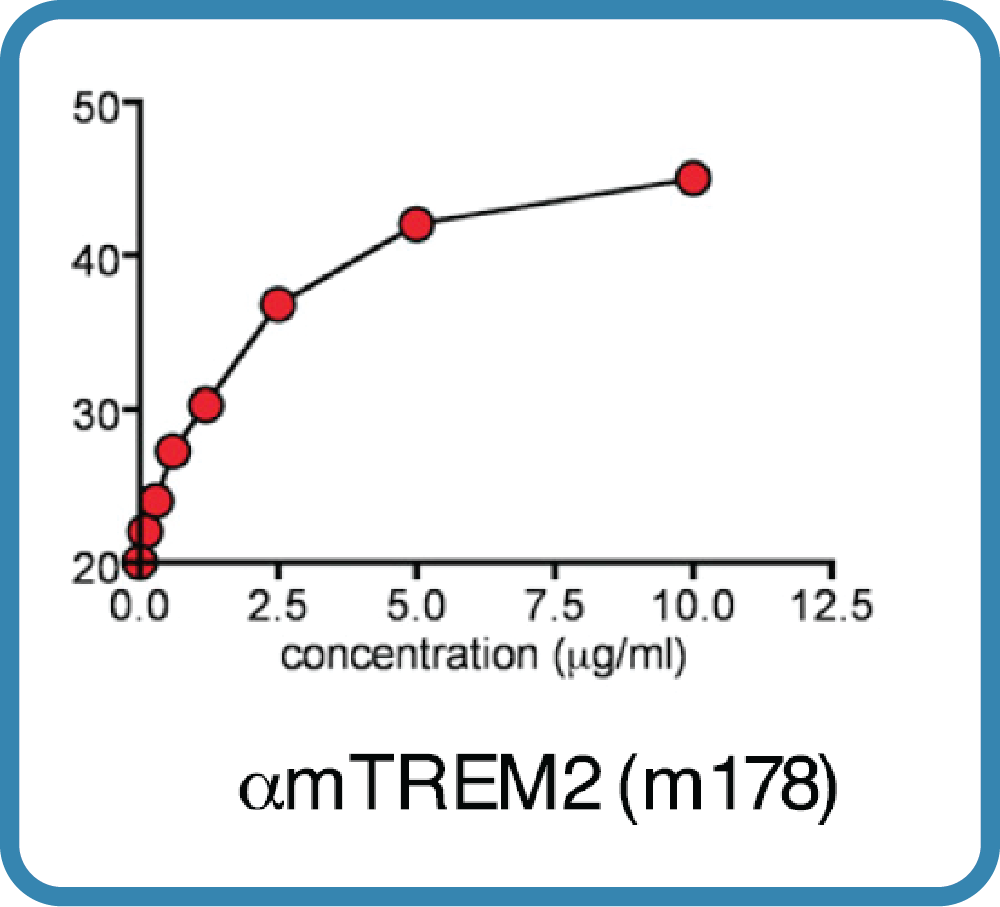
Antibody DetailsProduct DetailsReactive Species Mouse Host Species Mouse Expression Host HEK-293 Cells Recommended Isotype Controls Recommended Dilution Buffer Immunogen ectodomain of TREM2 Product Concentration ≥ 5.0 mg/ml Endotoxin Level <0.5 EU/mg as determined by the LAL method Purity ≥95% by SDS Page ⋅ ≥95% monomer by analytical SEC Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. State of Matter Liquid Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using only in vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Regulatory Status Research Use Only Country of Origin USA Shipping 2 - 8°C Wet Ice Additional Applications Reported In Literature ? FA, B, ELISA, FC Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity 178 (LALAPG) activity is directed against mouse TREM2. Background TREM2 is a transmembrane receptor in the immunoglobulin superfamily which has a short cytosolic tail that lacks signal transduction and trafficking motifs1. TREM2 initiates intracellular signaling by interacting with the adaptor proteins DNAX activation protein 12 (DAP12) and DAP10, which are phosphorylated to recruit signal transduction machinery when ligands bind. TREM2 interacts with a wide array of anionic ligands, including bacterial products such as lipopolysaccharide and dextran sulfates, DNA, lipoproteins, apolipoproteins, phospholipids1 and amyloid-β oligomers2.
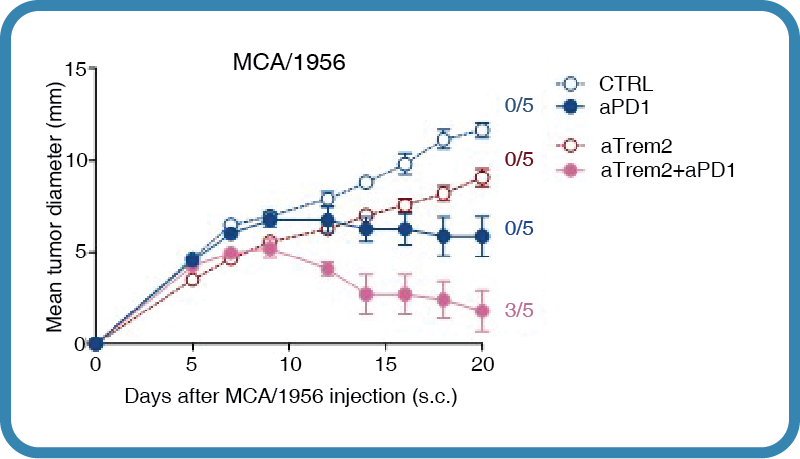
In healthy tissues, TREM2 is involved in tissue development and maintenance, synaptic pruning1, central nervous system homeostasis2, the hair follicle stem cell niche1, and activates immune remodeling when tissue damage is detected1. When dysregulated, TREM2 affects a variety of pathologies including neurodegeneration, fatty liver disease, obesity, atherosclerosis, and tumor microenvironment and development. In Alzheimer’s Disease, TREM2 activation initiates a signaling loop that promotes its own ligand production, sustains microglial responses, and leads to disease progression1, 2, 3. Defects in TREM2 also cause polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL), a fatal disease of pre-senile dementia1. Additionally, Trem2 knockout mice are more resistant to cancer growth and are more responsive to anti-PD1 immunotherapy than wild-type mice3. Given its broad role in pathology, TREM2 is a target of immunotherapy. Clone 178 was generated by immunizing a Wister rat with a recombinant protein consisting of the ectodomain of TREM2 fused to the human Ig constant domain4. Spleen cells were harvested, fused with Sp2/0 myeloma cells, and the resulting hybridomas screened against Jurkat cells transiently infected with TREM2. A recombinant form of 178 was then generated, in which the variable region of the heavy chain was grafted onto a mouse IgG2a constant region backbone containing a mutated Fc domain (LALAPG)3. The LALAPG mutation prevents recognition by Fc receptors and complement, thereby minimizing antibody-dependent cellular cytotoxicity and antibody-dependent phagocytosis. Clone 178 blocks ligand binding to TREM23 and does not cross-react with TREM14. Antigen Distribution In healthy tissues, TREM2 is expressed on myeloid cells (microglia and osteoclasts), infiltrating macrophages, and a small set of tissue-specific macrophages in the brain, adipose, adrenal gland, skin, alveola, endometrium, and placenta. TREM2 is also expressed by tumor-infiltrating macrophages. Ligand/Receptor DAP12 NCBI Gene Bank ID UniProt.org Q99NH8 Research Area Innate Immunity . Neuroscience Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. In Vivo Applications of Clone 178 (LALAPG) in MiceClone 178 (LALAPG) refers to a recombinant, Fc-muted (LALAPG) monoclonal antibody specific for mouse TREM2 (Triggering Receptor Expressed on Myeloid cells 2). Its engineered Fc region prevents effector functions such as antibody-dependent cellular cytotoxicity (ADCC) and complement activation, allowing it to block TREM2 signaling without depleting TREM2-expressing cells in vivo. Common In Vivo ApplicationsCancer Immunotherapy Research Neuroimmunology and Neurodegenerative Disease Models General Immunology and Myeloid Cell Biology Key Features Enabling In Vivo Use
Example ProtocolA typical in vivo protocol involves intraperitoneal (i.p.) injection of the antibody (e.g., 200 μg/mouse every 5 days) starting shortly after tumor implantation, allowing longitudinal assessment of tumor growth and immune modulation. Summary Table: In Vivo Applications of Clone 178 (LALAPG)
Clone 178 (LALAPG) is thus a versatile, mechanism-focused tool for in vivo studies of TREM2 biology across multiple disease models, particularly where distinguishing signaling blockade from cell depletion is critical. Other commonly used antibodies or proteins in the literature with 178 (LALAPG) include wild-type IgG1, LALA mutants, aglycosylated IgGs, and various other combinatorial Fc mutants. Key protein/antibody variants used with or for comparison to 178 (LALAPG):
Additional context:
These variants are frequently used in functional assays (e.g., blocking, receptor-binding, in vivo models) to investigate the biological role of Fc interactions, specificity, and therapeutic efficacy. Key findings from scientific citations of clone 178 (LALAPG) primarily highlight its use as a specialized research antibody for selective blockade of TREM2 function in studies of immunology, neurodegeneration, and cancer biology, particularly with minimized immune effector functions. Essential context and supporting details:
In summary, clone 178 (LALAPG) is recognized as a critical tool for dissecting TREM2 biology, especially where Fc-mediated mechanisms would otherwise confound interpretation. It has enabled advances in understanding TREM2 in neurodegenerative disease models and tumor immunology, often serving as the standard for TREM2 functional blockade in murine studies. Dosing Regimens of Clone 178 (LALAPG) in Mouse ModelsClone 178 (LALAPG) is a monoclonal antibody targeting mouse TREM2, engineered with an Fc-muted LALA-PG mutation to reduce effector functions such as FcγR binding. While specific studies using this exact clone are limited, available data and manufacturer guidelines provide insights into dosing variability. Standard Reported Dose
Variability Across Disease Models
General Guidance for Antibody Dosing in Mice
Summary Table
Key Points
References & Citations1. Deczkowska A, Weiner A, Amit I. Cell. 181(6):1207-1217. 2020.
2. Zhao P, Xu Y, Fan X, et al. MAbs. 14(1):2107971. 2022. 3. Molgora M, Esaulova E, Vermi W, et al. Cell. 182(4):886-900.e17. 2020. 4. Turnbull IR, Gilfillan S, Cella M, et al. J Immunol. 177(6):3520-3524. 2006. 5. Keshari, S, et al. Cell Reports, Volume 43, Issue 11, 114875. 2024. Technical ProtocolsCertificate of Analysis |
Related Products
Prod No. | Description |
|---|---|
D230 | |
I-1241 |
Formats Available
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.