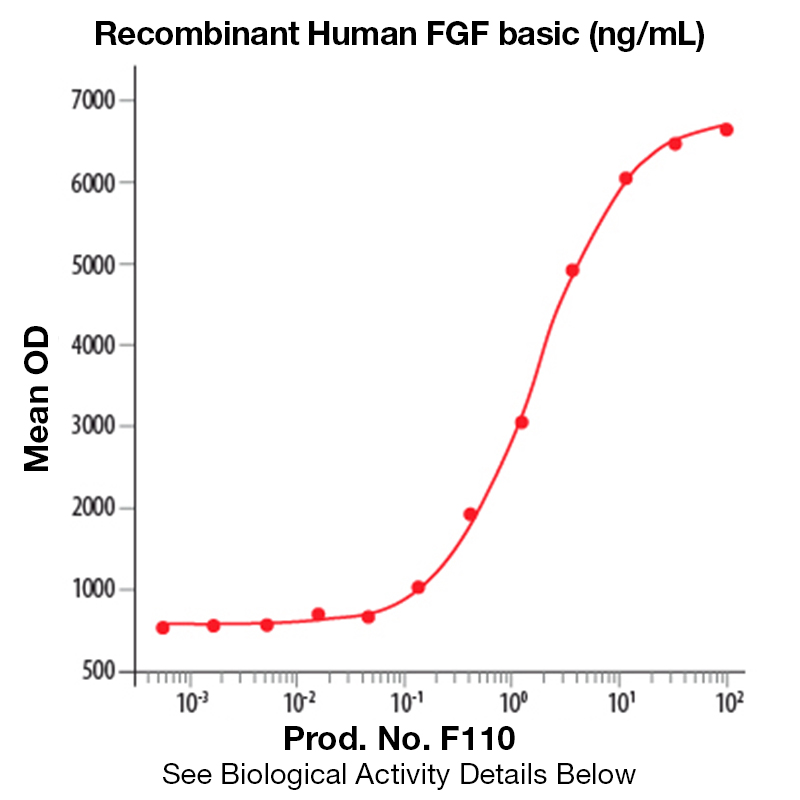
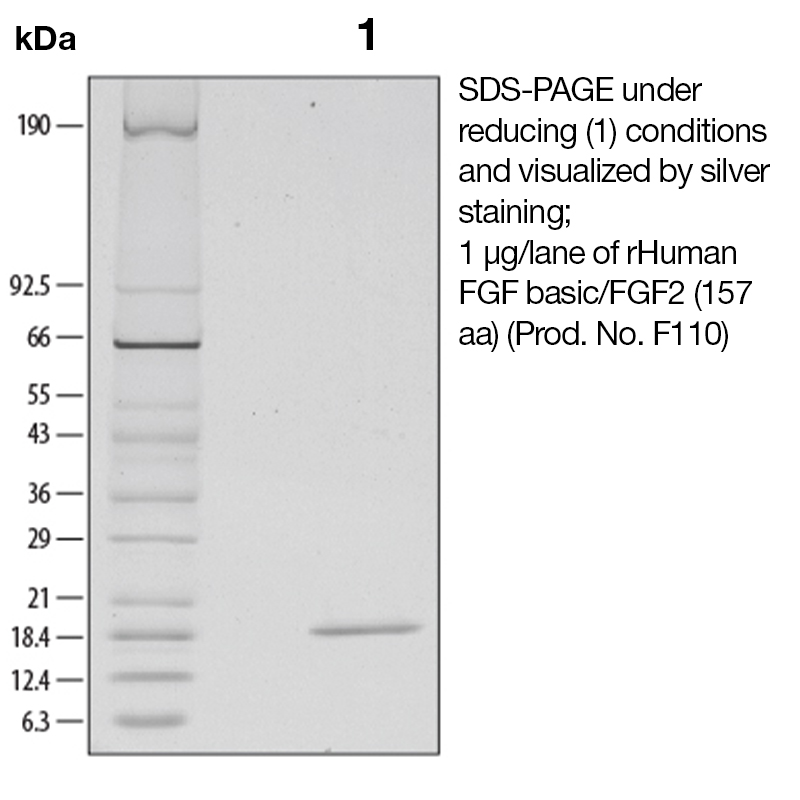
Recombinant Human FGF-Basic
Data
BackgroundBasic fibroblast growth factor (bFGF), also known as FGF-2 and FGF-β, is a non-glycosylated heparin binding growth factor and member of the FGF family of mitogenic proteins. Members of this family play a central role during prenatal development, postnatal growth and regeneration of a variety of tissues, by promoting cellular proliferation and differentiation (1). bFGF is expressed in the brain, pituitary, kidney, retina, bone, testis, adrenal gland, liver, placenta, and monocytes, epithelial and endothelial cells. It is secreted by mechanisms other than the classical protein secretion pathway due to the lack of a signal peptide. Acidic FGF (aFGF) and bFGF bind to the same high affinity receptors (2). Binding of bFGF to heparin or cell surface heparan sulfate proteoglycans is a prerequisite for ligation of bFGF to these receptors. aFGF and BFGF have a similar range of biological activities which are implicated in several important physiological and pathological processes, such as embryonic development and differentiation, morphogenesis, angiogenesis, and wound healing (3-4). Protein DetailsPurity >97% by SDS-PAGE and analyzed by silver stain. Endotoxin Level <0.1 EU/µg as determined by the LAL method Biological Activity The biological activity of Human FGF-basic was monitored in a mitogenic assay by measuring the FGF basic dependent <sup>3</sup>H-thymidine incorporation in quiescent NR6R-3T3 fibroblasts (Rizzino, A. et al., 1988. Cancer Research 48:4266 - 4271). The expected ED<sub>50</sub> for this effect is typically 0.1 - 0.25 ng/ml. Protein Accession No. NP_001997 Amino Acid Sequence gtmaagsit tlpalpedgg sgafppghfk dpkrlyckng gfflrihpdg rvdgvreksd phiklqlqae ergvvsikgv canrylamke dgrllaskcv tdecffferl esnnyntyrs rkytswyval krtgqyklgs ktgpgqkail flpmsaks
N-terminal Sequence Analysis Gly132 State of Matter Solution Predicted Molecular Mass The predicted molecular weight of Recombinant Human FGF-Basic is Mr 17.4 kDa. However, the actual molecular weight as observed by migration on SDS-PAGE is 19 kDa (reducing conditions). Predicted Molecular Mass 17.4 Formulation This recombinant protein solution was 0.2 µm filtered and formulated in Tris and NaCl. Storage and Stability This protein is stable for > twelve months when stored at -20°C to -70°C. After thawing and addition of a carrier protein, this protein may be stored at 2°C to 8°C for one month or for up to three months at -20°C to -70°C in a manual defrost freezer. Avoid Repeated Freeze Thaw Cycles. See Product Insert for exact lot specific storage instructions. Country of Origin USA Shipping Next Day 2-8°C NCBI Gene Bank Leinco Protein AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Recombinant Human FGF-Basic (FGF-2/bFGF) is a versatile growth factor that offers significant advantages across multiple research and therapeutic applications due to its broad biological activity and well-characterized mechanisms of action. Cellular Proliferation and DifferentiationFGF-Basic possesses broad mitogenic activity and stimulates proliferation across diverse cell types, including fibroblasts, myoblasts, osteoblasts, neuronal cells, endothelial cells, keratinocytes, chondrocytes, astrocytes, oligodendrocytes, and smooth muscle cells. Beyond proliferation, it induces neuronal differentiation, survival, and regeneration, making it particularly valuable for studies involving neural development and function. The protein also significantly promotes the proliferation of adipose-derived mesenchymal cells and enhances chondrogenesis in three-dimensional micromass culture. Tissue Regeneration and Wound HealingFGF-Basic demonstrates remarkable effectiveness in promoting tissue regeneration through multiple complementary mechanisms. It maintains osteoblast precursors in a proliferative state while simultaneously promoting angiogenesis, which is crucial for nutrient and oxygen supply in newly formed tissue. The protein stimulates secretion of other growth factors, such as vascular endothelial growth factor and hepatocyte growth factor, further enhancing vascular development and creating a favorable microenvironment for regeneration. In bone regeneration applications specifically, optimal efficacy has been demonstrated at a 0.3% concentration, which has received formal regulatory approval for periodontal regenerative medicine. Chronic wound research represents one of the largest areas of FGF-2 investigation, as these wounds often have reduced endogenous FGF-2 concentrations. Angiogenic ActivityFGF-Basic exhibits potent angiogenic activity and plays a key role in both physiological and pathological conditions. This property makes it essential for studying vascular development, wound repair, inflammation, and tumor biology. The protein binds to a family of four distinct, high-affinity tyrosine kinase receptors (FGFR-1 to FGFR-4) and also interacts with αvβ3 integrin, enabling multiple signaling pathways. Diverse Research ApplicationsThe protein's versatility extends to numerous research contexts, including embryonic development, stem cell maintenance, organoid culture, and disease modeling. It has proven valuable in bioassay applications across whole cell systems and organoid models, supporting investigations ranging from basic cell biology to translational research. Yes, recombinant human FGF-basic (FGF2) can be used as a standard for quantification or calibration in ELISA assays, provided it is compatible with your assay system. Multiple sources confirm that recombinant FGF-basic is routinely used as a standard in commercial ELISA kits and for assay calibration. Key considerations and supporting details:
Best Practices:
Summary Table: Use of Recombinant FGF-basic as ELISA Standard
In conclusion: Recombinant Human FGF-Basic (FGF2/bFGF) has been validated in published research for a wide range of applications, primarily in cell culture, bioassays, tissue engineering, regenerative medicine, and therapeutic studies. Key validated applications include:
Summary Table of Validated Applications
Additional Notes:
If you require protocol details or specific assay conditions for any application, please specify the intended use. To reconstitute and prepare Recombinant Human FGF-Basic (FGF-2/bFGF) protein for cell culture experiments, follow these best-practice steps to ensure protein stability and bioactivity: 1. Preparation Before Reconstitution
2. Reconstitution
3. Aliquoting and Storage
4. Working Solution Preparation
5. Additional Notes
Summary Table: Key Parameters for FGF-Basic Reconstitution
These guidelines will help ensure maximum stability and biological activity of recombinant human FGF-Basic for cell culture experiments. References & Citations1. Swain, JL. et al. (1991) Developement 111: 741 2. Grevers, G. et al. (1997) Laryngorhinootologie 76: 421 3. Bühring, HJ. et al. (2007) Differentiation. 75(4):279-91 Certificate of AnalysisIMPORTANT Use lot specific datasheet for all technical information pertaining to this recombinant protein. |
Related Products
Prod No. | Description |
|---|---|
F149 | |
F1105 | |
F107 | |
F110 | |
F1022 | |
F1027 |
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.