Anti-Mouse CTLA-4 [Clone 9D9] — Purified in vivo PLATINUM™ Functional Grade
Anti-Mouse CTLA-4 [Clone 9D9] — Purified in vivo PLATINUM™ Functional Grade
Product No.: C2856
Clone 9D9 Target CTLA-4 Formats AvailableView All Product Type Monoclonal Antibody Alternate Names CD152, Cytotoxic T Lymphocyte-Associated Antigen-4, Ly-56 Isotype Mouse IgG2b Applications FA , in vivo , WB |
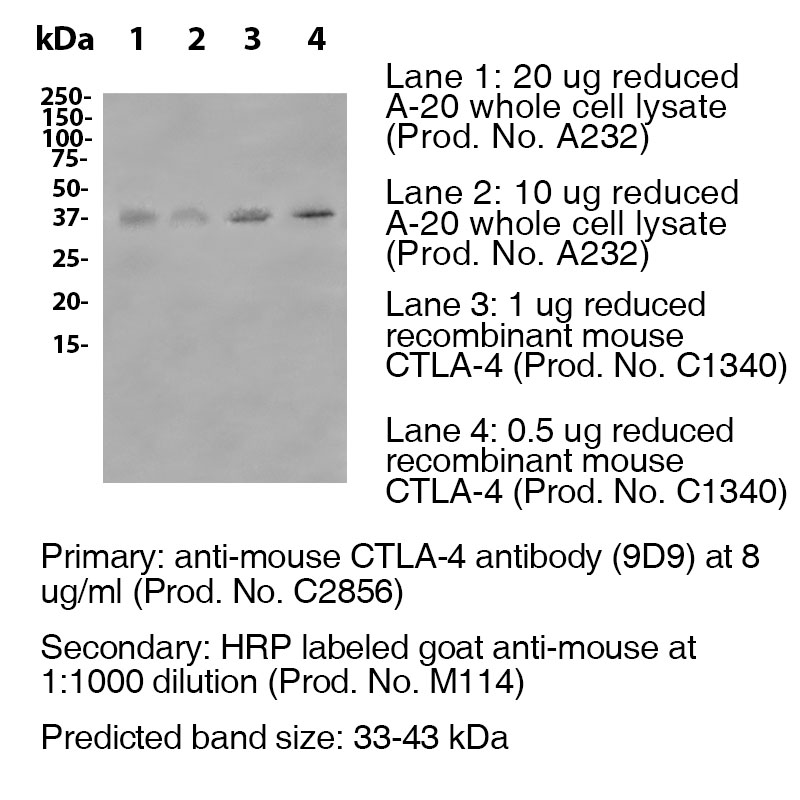
Data
Antibody DetailsProduct DetailsReactive Species Mouse Host Species Mouse Recommended Isotype Controls Recommended Isotype Controls Recommended Dilution Buffer Immunogen Not Available Product Concentration ≥ 5.0 mg/ml Endotoxin Level <0.5 EU/mg as determined by the LAL method Purity ≥98% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using in vitro cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Pathogen Testing To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s Purified Functional PLATINUM™ antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Next Day 2-8°C RRIDAB_2829611 Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Clone 9D9 recognizes an epitope on mouse CTLA-4. Background CTLA-4 is a 33 kD member of the Ig superfamily similar to CD28 in amino acid sequence, structure, and genomic organization. CTLA-4 is a protein receptor that functions as an immune checkpoint and downregulates immune responses. It is involved in the development of protective immunity and thymocyte regulation, in addition to the induction and maintenance of immunological tolerance. CTLA-4 has therapeutic potential both as an agonist to reduce immune activity, and an antagonist to increase immune activity. Antigen Distribution CTLA-4 is expressed on activated T and B lymphocytes. Ligand/Receptor CD80 (B7.1), CD86 (B7.2) Function Negative regulator of T cell activation NCBI Gene Bank ID UniProt.org Research Area Immunology . Inhibitory Molecules Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Common In Vivo Applications of Clone 9D9 in MiceClone 9D9 is a monoclonal antibody targeting mouse CTLA-4 (CD152), a key immune checkpoint that negatively regulates T cell activation. Its in vivo applications are primarily in the field of cancer immunotherapy, tumor microenvironment research, and mechanistic immunology studies in mice. Cancer Immunotherapy
Tumor Microenvironment Studies
Alternative Delivery Methods
Additional Applications
Summary Table: Key In Vivo Applications of 9D9
ConclusionClone 9D9 is a cornerstone tool in mouse immunology and cancer research, primarily for studying CTLA-4 blockade, enhancing anti-tumor immunity, modulating the tumor microenvironment, and testing novel antibody delivery platforms. Its ability to deplete Tregs and promote CD8+ T cell activity makes it especially valuable for understanding and improving cancer immunotherapy strategies. In the literature, antibodies or proteins commonly used alongside the 9D9 monoclonal antibody, which targets mouse CTLA-4, include:
These antibodies and proteins are used in various research contexts, including cancer immunotherapy models, to better understand the mechanisms of CTLA-4 blockade and to develop more effective treatments. Clone 9D9 is a widely used anti-mouse CTLA-4 monoclonal antibody with significant impact in immuno-oncology research. Key findings from scientific literature on 9D9 reveal that:
In summary, clone 9D9’s unique capability to combine CTLA-4 antagonism with effective Treg depletion leads to enhanced T cell activation and robust antitumor immunity, supporting its use in studies of immune checkpoint blockade and cancer immunotherapy. Dosing regimens of clone 9D9 (anti-CTLA-4) show remarkable consistency across different mouse models, though specific applications have introduced some variations in administration routes and frequencies. The standard dosing approach for clone 9D9 uses 100-250 μg per mouse administered via intraperitoneal injection every 3 days. This range has become the established protocol across most cancer immunotherapy studies. The specific dose within this range often depends on the experimental objectives and tumor model being studied. Route of AdministrationWhile intraperitoneal injection represents the primary delivery method, clone 9D9 has also been tested via intratumoral administration in certain experimental designs. This flexibility in delivery routes reflects the antibody's versatility in different research contexts, though intraperitoneal remains the most commonly reported approach. Timing and ScheduleThe every-3-day dosing schedule appears consistently across various mouse models. This frequency has been optimized to maintain therapeutic antibody levels while allowing for effective checkpoint blockade. In some protocols, treatment begins as early as 3 days after tumor implantation, demonstrating the antibody's utility in early-stage disease models. Combination Therapy ContextsClone 9D9 is frequently employed in combination therapy studies, where it's paired with anti-PD-1 or anti-PD-L1 antibodies. In these combination regimens, the dosing typically remains within the standard 100-250 μg range. Multiple-dose pharmacokinetic studies have shown that when administered at 1-10 mg/kg twice weekly, clone 9D9 demonstrates a notably long terminal half-life, approximately five-fold longer than some comparator antibodies, leading to greater than two-fold accumulation over repeated dosing cycles. Unique Functional PropertiesAn important consideration for clone 9D9 across different models is its dual mechanism of action. Due to its mouse IgG2b Fc domain, this clone not only blocks CTLA-4 but also depletes intratumoral regulatory T cells (Tregs), which enhances its anti-tumor immunity effects. This property distinguishes it from other anti-CTLA-4 clones and may influence dosing decisions in specific experimental contexts. References & CitationsTechnical ProtocolsCertificate of Analysis |
Related Products
Prod No. | Description |
|---|---|
C1748 | |
C2445 | |
C1614 | |
C2855 | |
C2860 | |
C2856 | |
C1319 | |
C1340 |
Formats Available
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.