Anti-Rhinovirus 16 [Clone R16-7]
Anti-Rhinovirus 16 [Clone R16-7]
Product No.: 18758
- -
- -
Clone R16-7 Target Rhinovirus 16 Formats AvailableView All Product Type Monoclonal Isotype Mouse IgG2b Applications IHC , WB |
Data
- -
- -
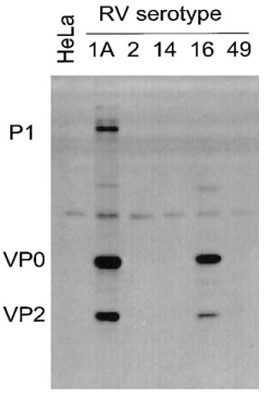
Antibody DetailsProduct DetailsReactive Species Rhinovirus 16 Host Species Mouse Immunogen Purified HRV16 Product Concentration Lot Specific Formulation This monoclonal antibody is formulated in phosphate buffered saline (PBS) pH 7.2 - 7.4 with no carrier protein or preservatives added. State of Matter Liquid Product Preparation Antibodies are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Storage and Handling Upon initial thawing, appropriately aliquot and store at -80°C. For long-term storage, keep at -80°C. Avoid repeated freeze-thaw cycles. Country of Origin USA Shipping Next Day 2-8°C Applications and Recommended Usage? Quality Tested by Leinco Immunoblotting: use at 2-5ug/mL.
Immunohistochemistry: use at 1-10ug/mL. These are recommended concentrations; Endusers should determine optimal concentrations for their applications. Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity This non-neutralizing antibody recognizes capsid protein VP2 (mw ~30kDa) of HRV16, HRV1A, and HRV39 and VP2 precursors VP0 (mw ~37kDa) and P1 (mw ~90kDa). Background Picornaviruses are small, non-enveloped RNA viruses with an icosahedral capsid and a single strand, plus-sense RNA genome. The genome encodes a single polyprotein that is proteolytically processed by viral proteases into structural and non-structural proteins. The family of picornaviruses includes numerous human and animal viruses including more than 100 s of human rhinoviruses (HRV). HRV infections are characteristic upper airway infections (the main cause of the common cold), and they provoke significant lower airway symptoms for patients with asthma, cystic fibrosis, or chronic obstructive pulmonary disease. Research Area Infectious Disease References & Citations1) Mosser AG et al. 2002 J Infect Dis 185: 734. 2) Mosser AG et al. 2005 Am J Respir Crit Care Med 171: 645. 3) Jurgeit A et a. 2010 Virology J 7: 264. 4) Chattoraj SS et al. 2011 Infect Immun 79: 4131. Technical ProtocolsCertificate of Analysis |