Anti-Amyloid Oligomerαβ [Polyclonal]
Anti-Amyloid Oligomerαβ [Polyclonal]
Product No.: 57006
- -
- -
Target Amyloid Oligomerαβ Product Type Polyclonal Alternate Names APP, ABPP, APPI, Alzheimer disease amyloid A4 protein homolog, Alzheimer disease amyloid protein, Amyloid precursor protein, Amyloid-β Applications ELISA , ICC , IF , IHC , IP , WB |
Data
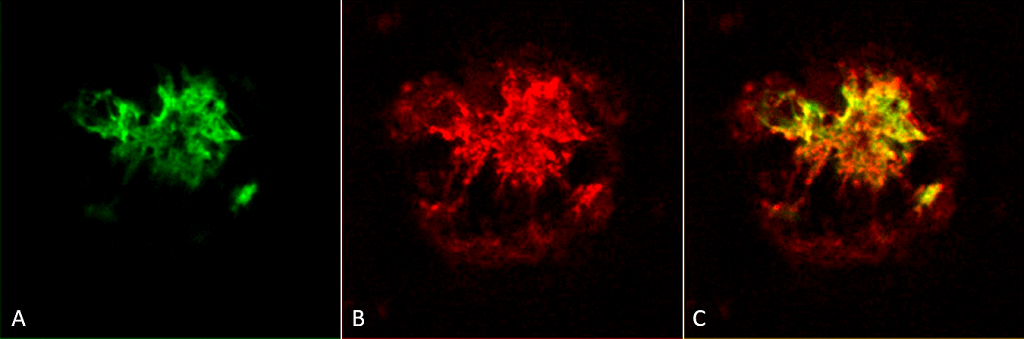 Immunohistochemistry analysis using Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006). Tissue: Alzheimer’s Disease brain. Species: Human. Fixation: Formalin fixed. Primary Antibody: Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006) at 1:1000. Secondary Antibody: Goat Anti-Rabbit ATTO 594 (red). Localization: Plaque. (A) Amyloid Fibril (OC) Antibody (57005). (B) Amyloid Oligomer (A11) Antibody (57006). (C) Composite. Courtesy of: Dr. Elizabeth Head, University of California, Irvine.
Immunohistochemistry analysis using Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006). Tissue: Alzheimer’s Disease brain. Species: Human. Fixation: Formalin fixed. Primary Antibody: Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006) at 1:1000. Secondary Antibody: Goat Anti-Rabbit ATTO 594 (red). Localization: Plaque. (A) Amyloid Fibril (OC) Antibody (57005). (B) Amyloid Oligomer (A11) Antibody (57006). (C) Composite. Courtesy of: Dr. Elizabeth Head, University of California, Irvine.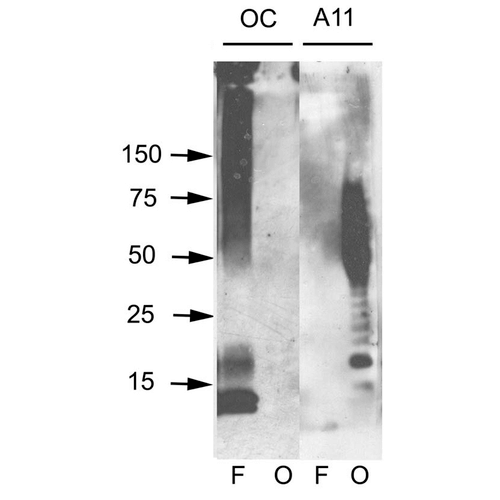 Western blot analysis of Human Abeta42 fibrils and prefibrillar oligomers showing detection of Amyloid Oligomers (A11) protein using Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006). Primary Antibody: Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006) at 1:1000. Courtesy of: Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., et al. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. doi: 10.1126/science.1079469.
Western blot analysis of Human Abeta42 fibrils and prefibrillar oligomers showing detection of Amyloid Oligomers (A11) protein using Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006). Primary Antibody: Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006) at 1:1000. Courtesy of: Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., et al. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. doi: 10.1126/science.1079469.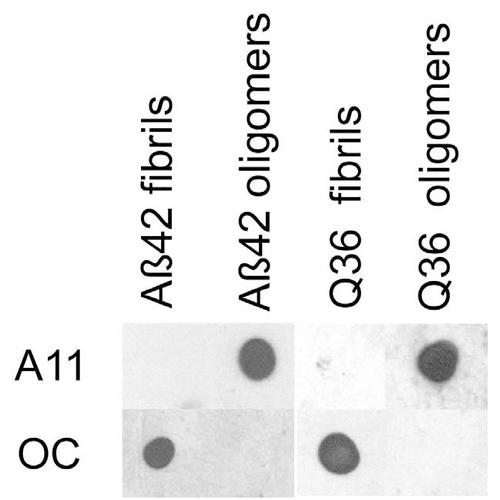 Dot blot analysis using Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006). Tissue: Abeta42 fibrils and prefibrillar oligomers. Species: Human. Primary Antibody: Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006) at 1:1000. Courtesy of: Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., et al. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. doi: 10.1126/science.1079469.
Dot blot analysis using Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006). Tissue: Abeta42 fibrils and prefibrillar oligomers. Species: Human. Primary Antibody: Rabbit Anti-Amyloid Oligomers (A11) Polyclonal Antibody (57006) at 1:1000. Courtesy of: Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., et al. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. doi: 10.1126/science.1079469. - -
- -
Antibody DetailsProduct DetailsReactive Species Human ⋅ Mouse ⋅ Rat Host Species Rabbit Immunogen Synthetic molecular mimic of soluble oligomers. Product Concentration 0.5 mg/ml Formulation PBS, pH 7.0, 0.09% sodium azide and 50% glycerol. State of Matter Liquid Product Preparation Purified by immunoaffinity chromatography Storage and Handling This product is stable for at least 1 year at -20°C. Freeze in multiple aliquots to avoid repeated freeze-thaw cycles. Regulatory Status For in vitro investigational use only. Not for
use in therapeutic or diagnostic procedures. Country of Origin USA Shipping Next Day 2-8°C Applications and Recommended Usage? Quality Tested by Leinco Immunoblotting: use at 0.5-1ug/mLDot blot: use at 0.5-1ug/
mLELISA: use at 1-10ug/mL with amyloid oligomer-containing samples on the solid phase. These are recommended concentrations. Enduser should determine optimal concentrations for their applications. Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity This antibody recognizes a peptide
backbone epitope that is common to all
types of amyloid oligomers but is not found
in native proteins, amyloidogenic monomers,
or mature amyloid fibrils. Background Amyloid monomeric proteins can oligomerize into destructive amyloid fibrils. Amyloidogenic conformations of non- disease related proteins can be created by partial protein misfolding or denaturation. Many degenerative diseases are known to be related to the accumulation of misfolded proteins as amyloid fibers. These include the amyloid-beta peptide plaques and tau neurofibrillary tangles in senile plaques of Alzheimer's symptomology, the deposition of alpha-synuclein in the Lewy bodies of Parkinson's disease, and accumulation of polyglutamine-containing aggregates in Huntington's disease. Function Functions as a cell surface receptor and performs physiological functions on the surface of neurons relevant to neurite growth, neuronal adhesion and axonogenesis. Interaction between APP molecules on neighboring cells promotes synaptogenesis (PubMed:25122912). Involved in cell mobility and transcription regulation through protein-protein interactions. Can promote transcription activation through binding to APBB1-KAT5 and inhibits Notch signaling through interaction with Numb. Couples to apoptosis-inducing pathways such as those mediated by G(o) and JIP. Inhibits G(o) alpha ATPase activity (By similarity). Acts as a kinesin I membrane receptor, mediating the axonal transport of beta-secretase and presenilin 1 (By similarity). By acting as a kinesin I membrane receptor, plays a role in axonal anterograde transport of cargo towards synapes in axons (PubMed:17062754, PubMed:23011729). Involved in copper homeostasis/oxidative stress through copper ion reduction. In vitro, copper-metallated APP induces neuronal death directly or is potentiated through Cu(2+)-mediated low-density lipoprotein oxidation. Can regulate neurite outgrowth through binding to components of the extracellular matrix such as heparin and collagen I and IV. The splice isoforms that contain the BPTI domain possess protease inhibitor activity. Induces a AGER-dependent pathway that involves activation of p38 MAPK, resulting in internalization of amyloid-beta peptide and leading to mitochondrial dysfunction in cultured cortical neurons. Provides Cu(2+) ions for GPC1 which are required for release of nitric oxide (NO) and subsequent degradation of the heparan sulfate chains on GPC1. {UniProtKB:P12023, PubMed:17062754, PubMed:23011729, PubMed:25122912}.; Amyloid-beta peptides are lipophilic metal chelators with metal-reducing activity. Bind transient metals such as copper, zinc and iron. In vitro, can reduce Cu(2+) and Fe(3+) to Cu(+) and Fe(2+), respectively. Amyloid-beta protein 42 is a more effective reductant than amyloid-beta protein 40. Amyloid-beta peptides bind to lipoproteins and apolipoproteins E and J in the CSF and to HDL particles in plasma, inhibiting metal-catalyzed oxidation of lipoproteins. APP42-beta may activate mononuclear phagocytes in the brain and elicit inflammatory responses. Promotes both tau aggregation and TPK II-mediated phosphorylation. Interaction with overexpressed HADH2 leads to oxidative stress and neurotoxicity. Also binds GPC1 in lipid rafts.; Appicans elicit adhesion of neural cells to the extracellular matrix and may regulate neurite outgrowth in the brain. {ECO:0000250}.; The gamma-CTF peptides as well as the caspase-cleaved peptides, including C31, are potent enhancers of neuronal apoptosis.; N-APP binds TNFRSF21 triggering caspase activation and degeneration of both neuronal cell bodies (via caspase-3) and axons (via caspase-6). NCBI Gene Bank ID UniProt.org Research Area Neuroscience . Alzheimer's Disease . Cell Signaling References & CitationsTechnical ProtocolsCertificate of Analysis |


