Anti-Human CD4 (Ibalizumab) [Clone TNX-355]
Anti-Human CD4 (Ibalizumab) [Clone TNX-355]
Product No.: LT3200
Product No.LT3200 Clone TNX-355 Target CD4 Product Type Biosimilar Recombinant Human Monoclonal Antibody Alternate Names CD4, CD4mut, CD4 molecule, OKT4D, IMD79 Isotype Human IgG4κ Applications ELISA , FA , FC , IHC , N , WB |
Data
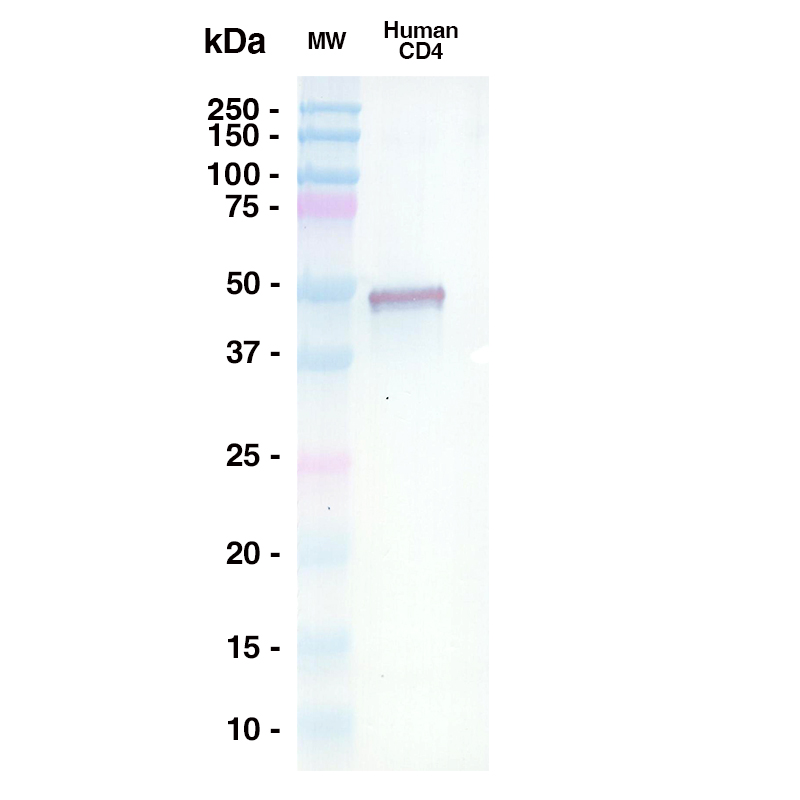 Western Blot
Western Blot
A 0.5ug sample of Human CD4 was loaded onto an SDS-PAGE gel under reducing conditions and probed with TNX-355 (Leinco Prod. No.: LT3200) @ 15ug/mL, Goat Anti-Human IgG secondary antibody 1:1000 dilution (Goat Anti-Human IgG (H+L) adsorbed against ms HRP (Leinco Prod. No.: H215)
Antibody DetailsProduct DetailsReactive Species Human Host Species Human Expression Host HEK-293 Cells FC Effector Activity Active Recommended Isotype Controls Immunogen Recombinant Human CD4 Product Concentration ≥ 5.0 mg/ml Endotoxin Level < 1.0 EU/mg as determined by the LAL method Purity ≥95% by SDS Page ⋅ ≥95% monomer by analytical SEC Formulation This biosimilar antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. State of Matter Liquid Product Preparation Recombinant biosimilar antibodies are manufactured in an animal free facility using only in vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Regulatory Status Research Use Only (RUO). Non-Therapeutic. Country of Origin USA Shipping 2-8°C Wet Ice Applications and Recommended Usage? Quality Tested by Leinco ELISA WB Additional Applications Reported In Literature ? FA IHC-FF FC N Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity This non-therapeutic biosimilar antibody uses the same variable region sequence as the therapeutic antibody Ibalizumab. This product is for research use only. Ibalizumab binds to domain 2 of CD4 T cell receptors, on the protein surface opposite where the major histocompatibility complex-class II and HIV-1 gp120 binding sites are located. Ibalizumab binds to both human and monkey CD4. Background CD4 is a cell surface glycoprotein essential for both T cell activation and human immunodeficiency virus type-1 (HIV-1) infection 1,2. CD4 consists of an extracellular segment composed of four tandem immunoglobulin-like domains (D1 to D4), a single transmembrane span, and a short C-terminal cytoplasmic tail 1. HIV-1 entry into host CD4 cells is a complex process that occurs through the interaction of HIV-1 glycoprotein 120 (gp120) with extracellular CD4 D1 3,4. When gp120 binds to CD4, a conformational shift occurs that allows co-receptors to bind to the gp120/receptor complex, leading to viral fusion and entry. Ibalizumab is the first CD4-directed post-attachment HIV-1 entry inhibitor and prevents entry of a broad spectrum of HIV-1 isolates 1,5,6,7,8.
Ibalizumab selectively binds to an epitope on CD4 D2 (residues 121-124 and 127-134 9 and especially L96, P121, P122, and Q163 1,7) as well as residues E77 and S79 on D1 at the interface between D1 and D2 4,7. Ibalizumab primarily contacts the BC-loop in D2 at the D1-D2 junction on the opposite side to the gp120 and major histocompatibility complex II (MHC-II) binding sites 2. Ibalizumab does not inhibit HIV-1 gp120 binding to D1, but instead induces conformational changes that via steric hindrance block gp120 and HIV co-receptors from interacting, thereby preventing viral fusion and entry 3,4,7,10,11. Additionally, because the cellular epitope is distant from the D1 MHC-II binding site 4, MHC-II mediated immunosuppression is prevented 3. Furthermore, as a humanized IgG4 antibody, ibalizumab displays low affinity for C1q and FcɣRI receptors of natural killer cells and consequently has low cellular cytotoxic dependent activity and no Fc-mediated CD4+ T cell depletion 3. Ibalizumab was derived from mu5A8 by grafting the mouse complementary-determining region onto a human IgG4 construct 1,3,12. The chemical name is immunoglobulin G4, anti-(human CD4 (antigen)) (human-mouse monoclonal 5A8 γ4-chain), disulphide with human-mouse monoclonal 5A8 κ-chain, dimer 5. Antigen Distribution CD4 is primarily found on T lymphocytes. Ligand/Receptor CD4/CD4 receptor NCBI Gene Bank ID UniProt.org Research Area Biosimilars . Cancer . HIV . Immunology . Pathology Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Research-grade Ibalizumab biosimilars can be used as calibration standards or reference controls in a pharmacokinetic (PK) bridging ELISA to measure drug concentration in serum samples by employing a precise and robust analytical strategy. Here's how they are utilized: Calibration and Reference Controls
PK Bridging ELISA
Pharmacokinetic Studies
ELISA Performance
By using biosimilar Ibalizumab as calibration standards or reference controls in PK bridging ELISAs, researchers can accurately measure drug concentrations, assess pharmacokinetics, and support regulatory submissions for biosimilar approval. The primary models where research-grade anti-CD4 antibodies are administered in vivo to study tumor growth inhibition and to characterize tumor-infiltrating lymphocytes (TILs) are syngeneic mouse tumor models. Humanized mouse models are less commonly used for anti-CD4 studies due to the species specificity of available antibodies and differences in immune repertoires. Details and context:
Additional Notes:
In summary, syngeneic mouse tumor models are the main systems in which research-grade anti-CD4 antibodies are administered in vivo to study tumor growth inhibition and TIL dynamics, while humanized models are less frequently used due to logistical and technical restrictions. Researchers use Ibalizumab biosimilars—non-therapeutic antibodies mirroring the clinical molecule—to study immune modulation in advanced immune-oncology models, often in parallel or combination with other checkpoint inhibitors like anti-CTLA-4 or anti-LAG-3 biosimilars to elucidate possible synergistic effects. Research Approach and Methodology
Example Workflow
Rationale
Limitations and Special Considerations
In summary, use of ibalizumab biosimilars in conjunction with other checkpoint inhibitor biosimilars in research settings allows detailed dissection of complex immune interactions, aids in identifying promising multi-target therapy strategies, and supports the optimization of next-generation immune-oncology combinatorial regimens. In a bridging anti-drug antibody (ADA) ELISA for immunogenicity testing, an Ibalizumab biosimilar can be used either as the capture or detection reagent because it mimics the structure of the therapeutic drug, enabling sensitive and specific detection of antibodies generated in patients against Ibalizumab. Key steps and rationale:
Summary table: Bridging ADA ELISA components
This setup enables monitoring of a patient’s immune response to the drug during therapy, fulfilling regulatory and clinical requirements for immunogenicity surveillance. References & Citations1. Song R, Franco D, Kao CY, et al. J Virol. 84(14):6935–6942. 2010. 2. Freeman MM, Seaman MS, Rits-Volloch S, et al. Structure. 18(12):1632–1641. 2010. 3. Iacob SA, Iacob DG. Front Microbiol. 8:2323. 2017. 4. Chahine EB, Durham SH. Ann Pharmacother. 55(2):230-239. 2021. 5. Markham A. Drugs. 78(7):781-785. 2018. 6. Reimann KA, Lin W, Bixler S, et al. AIDS Res Hum Retroviruses. 13(11):933-943. 1997. 7. Beccari MV, Mogle BT, Sidman EF, et al. Antimicrob Agents Chemother. 63(6):e00110-19. 2019. 8. Blair HA. Drugs. 80(2):189-196. 2020. 9. Burkly LC, Olson D, Shapiro R, et al. J Immunol. 149:1779–1787. 1992. 10. Moore JP, Sattentau QJ, Klasse PJ, et al. J Virol. 66(8):4784-4793. 1992. 11. Bettiker RL, Koren DE, Jacobson JM. Curr Opin HIV AIDS. 13(4):354-358. 2018. 12. Boon L, Holland B, Gordon W, et al. Toxicology. 172(3):191-203. 2002. 13. Reimann KA, Khunkhun R, Lin W, et al. AIDS Res Hum Retroviruses. 18(11):747-755. 2002. 14. Kuritzkes DR, Jacobson J, Powderly WG, et al. J Infect Dis. 189(2):286-291. 2004. 15. Jacobson JM, Kuritzkes DR, Godofsky E, et al. Antimicrob Agents Chemother. 53(2):450-457. 2009. 16. Toma J, Weinheimer SP, Stawiski E, et al. J Virol. 85(8):3872–3880. 2011. 17. Pace CS, Fordyce MW, Franco D, et al. J Acquir Immune Defic Syndr. 62(1):1–9. 2013. Technical ProtocolsCertificate of Analysis |
Related Products
Formats Available
Prod No. | Description |
|---|---|
LT3200 | |
LT3205 |
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.


