Anti-Human TNF-α Adalimumab [Clone D2E7]
Anti-Human TNF-α Adalimumab [Clone D2E7]
Product No.: LT100
Product No.LT100 Clone D2E7 Target TNF-α Product Type Biosimilar Recombinant Human Monoclonal Antibody Alternate Names DIF; TNFA; TNFSF2; TNLG1F; TNF-alpha Isotype Human IgG1κ Applications B , ELISA , FA , FC , IF , IHC , IP , N |
Data
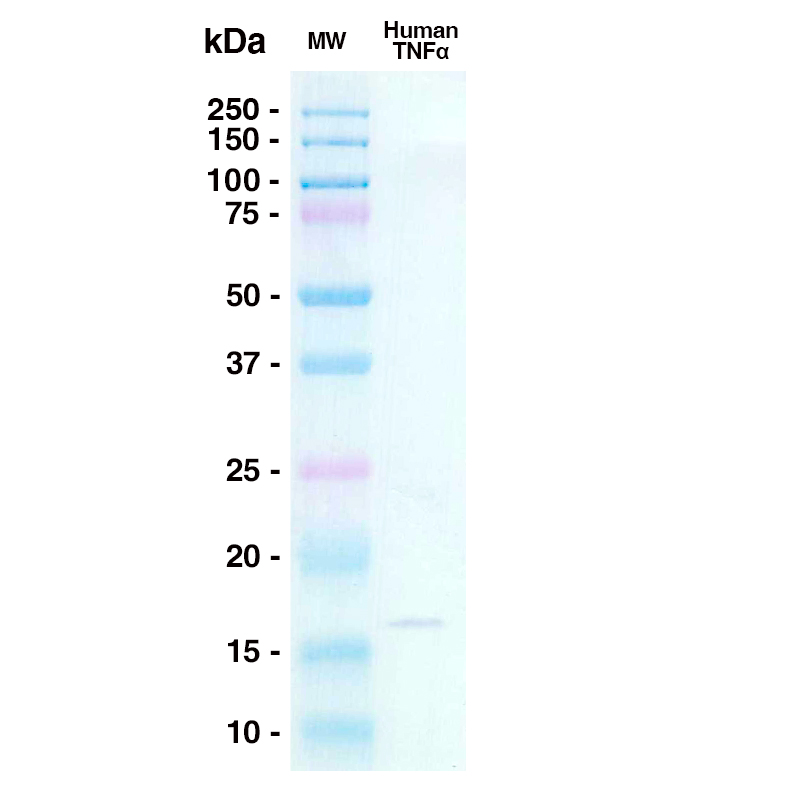 Western Blot
Western Blot
A 0.3ug sample of Human TNFa was loaded onto an SDS-PAGE gel under reducing conditions and probed with D2E7 (Leinco Prod. No.: LT100) @ 10ug/mL, Goat Anti-Human IgG secondary antibody 1:1000 dilution (Goat Anti-Human IgG (H+L) adsorbed against ms HRP Leinco Prod. No.: H215)
Antibody DetailsProduct DetailsReactive Species Human Host Species Human Expression Host HEK-293 Cells FC Effector Activity Active Immunogen Human TNF alpha Product Concentration ≥ 5.0 mg/ml Endotoxin Level < 1.0 EU/mg as determined by the LAL method Purity ≥98% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This biosimilar antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Recombinant biosimilar antibodies are manufactured in an animal free facility using only in vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Pathogen Testing To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s recombinant biosimilar antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Regulatory Status Research Use Only (RUO). Non-Therapeutic. Country of Origin USA Shipping 2-8°C Wet Ice RRIDAB_2893873 Applications and Recommended Usage? Quality Tested by Leinco FC The suggested concentration for Adalimumab biosimilar antibody for staining cells in flow cytometry is ≤ 0.25 μg per 106 cells in a volume of 100 μl. Titration of the reagent is recommended for optimal performance for each application. Additional Applications Reported In Literature ? N B ELISA FA IF IP IHC Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity This non-therapeutic biosimilar antibody uses the same variable region sequence as the therapeutic antibody Adalimumab. Clone D2E7 binds to soluble TNF- α, but not to TNF- β (lymphotoxin). This product is for research use only. Background Adalimumab is a research-grade monoclonal antibody that works by inactivating tumor necrosis factor-alpha (TNF-α). TNF-α is a 17.5 kD protein that mediates inflammation and immunity caused by the invasion of viruses, bacteria, and parasites by initiating a cascade of cytokines that increase vascular permeability, thus bringing macrophages and neutrophils to the site of infection. TNF-α secreted by the macrophage causes the blood to clot which provides containment of the infection. TNF-α inactivation has proven to be important in downregulating the inflammatory reactions associated with autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, moderate to severe chronic psoriasis, and juvenile idiopathic arthritis. Adalimumab blocks the interaction with the p55 and p75 cell surface TNF receptors thus, neutralizing the biological function of TNF. Anti-Human TNF alpha (Adalimumab) utilizes the same variable regions from the therapeutic antibody Adalimumab making it ideal for research projects. Antigen Distribution TNF-α is secreted by macrophages, monocytes, neutrophils, T cells, B cells, NK cells, LAK cells. PubMed NCBI Gene Bank ID UniProt.org Research Area Biosimilars Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Research-grade Adalimumab biosimilars are used as calibration standards or reference controls in pharmacokinetic (PK) bridging ELISAs by constructing a standard curve against which the unknown drug concentrations in serum samples are measured. This allows the precise quantification of adalimumab (originator or biosimilar) in patient samples and supports cross-comparisons between biosimilar and reference products. Key aspects:
Summary Table: Use of Adalimumab Biosimilars as Calibration Standards in PK Bridging ELISA
In summary, PK ELISA bridging studies rely on well-characterized biosimilar and/or originator adalimumab standards for accurate, reproducible drug quantification, enabling regulatory and clinical cross-comparison of biosimilar and reference products. The primary in vivo models where a research-grade anti-TNF-α antibody is administered to study tumor growth inhibition and characterize tumor-infiltrating lymphocytes (TILs) are syngeneic mouse models and, to a lesser extent, humanized mouse models. Key details:
Patient-derived xenograft (PDX) models are rarely used for direct anti-TNF-α mAb studies targeting the immune compartment, as these mice lack fully functional immune systems unless humanized. Summary: Current State of Adalimumab Biosimilar Use in Complex Immune-OncologyAdalimumab is a monoclonal antibody targeting tumor necrosis factor-alpha (TNF-α) and is widely used in autoimmune diseases. Its biosimilars—near-identical copies with similar efficacy and safety profiles—are increasingly available and studied in various contexts. However, there is no published evidence in the provided search results or in the literature that adalimumab or its biosimilars are being combined with checkpoint inhibitors (such as anti-CTLA-4 or anti-LAG-3 antibodies, or their biosimilars) in complex immune-oncology models to study synergistic effects. Synergistic Studies in Immune-Oncology: Focus on Checkpoint Inhibitor CombinationsCurrent research on immune-oncology combination therapies primarily explores combinations of immune checkpoint inhibitors (ICIs) themselves (e.g., anti-CTLA-4 plus anti-PD-1/PD-L1), or ICIs with chemotherapy, targeted therapies, radiotherapy, and other agents that modulate the tumor microenvironment. These combinations aim to overcome resistance, enhance T-cell infiltration, and improve clinical outcomes in various cancers.
Scientific Rationale for TNF-α Inhibition in Immuno-OncologyWhile TNF-α inhibitors like adalimumab are not standard in cancer immunotherapy, there is theoretical interest in their potential to modulate the tumor microenvironment and inflammation. However, clinical and preclinical evidence supporting the combination of TNF-α inhibitors with checkpoint inhibitors is lacking. In fact, TNF-α inhibitors are primarily used to manage immune-related adverse events (irAEs) from checkpoint inhibitors rather than as direct anti-cancer agents. Challenges and Considerations
ConclusionCurrent research in immune-oncology focuses on synergistic combinations of checkpoint inhibitors with each other or with other classes of anti-cancer agents, not with TNF-α inhibitors like adalimumab. There is no evidence from the literature that researchers are using adalimumab biosimilars in conjunction with checkpoint inhibitor biosimilars to study synergistic effects in complex immune-oncology models. Any future exploration of such combinations would require strong preclinical rationale, careful safety assessment, and rigorous clinical evaluation. In a bridging ADA ELISA for immunogenicity testing, a biosimilar Adalimumab can serve as both the capture and detection reagent to specifically measure anti-adalimumab antibodies (ADAs) generated in patients undergoing therapy with Adalimumab or its biosimilars. Key methodology details:
Why use a biosimilar as both capture and detection reagent?
Bridging ELISA advantages:
Context of patient monitoring:
Summary table: Biosimilar Adalimumab in bridging ADA ELISA
This approach represents a standardized and sensitive method for monitoring a patient’s immune response against therapeutic Adalimumab or its biosimilars during treatment. References & Citations1. Omidinia, E. et al. (2019) Protein Expr Purif. 155:59-65. Technical ProtocolsCertificate of Analysis |
Formats Available
Prod No. | Description |
|---|---|
LT100 | |
LT103 | |
LT104 | |
LT102 | |
LT101 | |
LT111 | |
LT106 | |
LT107 | |
LT105 |
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.


