Anti-Mouse CD137 (4-1BB) [Clone 3H3] — Purified in vivo PLATINUM™ Functional Grade
Anti-Mouse CD137 (4-1BB) [Clone 3H3] — Purified in vivo PLATINUM™ Functional Grade
Product No.: C2835
Clone 3H3 Target 4-1BB Formats AvailableView All Product Type Monoclonal Antibody Alternate Names CD137, CD137L, TNFSF9 Isotype Rat IgG2a Applications ELISA , FA , in vivo , WB |
Data
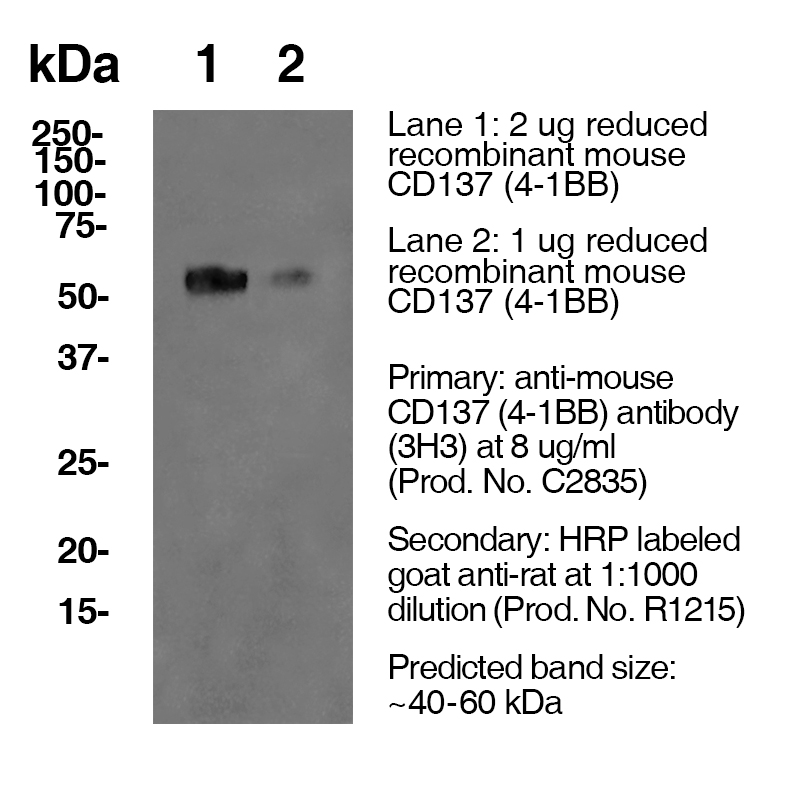
Antibody DetailsProduct DetailsReactive Species Mouse Host Species Rat Recommended Isotype Controls Recommended Isotype Controls Recommended Dilution Buffer Immunogen Recombinant Mouse CD137 human Fc fusion protein Product Concentration ≥ 5.0 mg/ml Endotoxin Level <0.5 EU/mg as determined by the LAL method Purity ≥98% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using in vitro cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Pathogen Testing To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s Purified Functional PLATINUM™ antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Next Day 2-8°C RRIDAB_2829593 Applications and Recommended Usage? Quality Tested by Leinco WB ELISA Additional Applications Reported In Literature ? in vivo 4-1BB stimulation in vitro 4-1BB stimulation Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Clone 3H3 recognizes an epitope on mouse 4-1BB. Background 4-1BB (CD137) is a 39 kD transmembrane protein that is a member of the tumor necrosis factor (TNF) receptor family and is a co-stimulatory molecule that plays a role in T-cell-mediated proliferative response. When binding its ligand, CD137 provides costimulatory signals to both CD4 and CD8 T cells via the activation of NF-B, c-Jun and p38 downstream pathways. Crosslinking of CD137 boosts T cell proliferation, IL-2 secretion, survival and cytolytic activity. Furthermore, it can increase immune activity to eliminate tumors in mice. Agonistic anti-CD137 antibodies have been reported to stimulate a more intense immune system attack on cancers. Antigen Distribution 4-1BB is expressed on activated B cells and T cells, macrophages, and dendritic cells. Ligand/Receptor 4-1BB (CDw137) NCBI Gene Bank ID UniProt.org Research Area Costimulatory Molecules . Immunology Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Clone 3H3 is primarily used as an agonistic monoclonal antibody targeting mouse CD137 (4-1BB), with several important in vivo applications in mouse research models. Cancer Immunotherapy StudiesThe predominant application of clone 3H3 is in cancer immunotherapy research. This antibody stimulates 4-1BB signaling to enhance anti-tumor immunity, particularly by activating CD8+ T cell responses. When administered in vivo, 3H3 has been shown to delay tumor growth in mouse models. The mechanism involves inducing more effector molecules from CD8+ T cells, increasing their proliferation, and decreasing apoptosis, all of which contribute to a more robust anti-tumor immune response. Immune Modulation ResearchBeyond cancer models, clone 3H3 is widely used to investigate immune modulation in infectious disease models. The antibody works by providing costimulatory signals to both CD4 and CD8 T cells, boosting T cell proliferation, IL-2 secretion, survival, and cytolytic activity. This makes it valuable for studying how enhancing T cell responses can combat various pathogens. T Cell Activation StudiesResearchers utilize 3H3 to study T cell-mediated immune responses more broadly. The antibody's agonistic properties make it an effective tool for investigating how 4-1BB costimulation regulates CD28 co-stimulation in targeted cell subsets and favors Th1 development while maintaining long-term cell growth. It also helps researchers understand the duration and immunogenicity of dendritic cell-T cell interactions. The antibody is typically administered at doses determined by individual research protocols, with functional grade preparations containing endotoxin levels ≤1.0 EU/mg to ensure minimal confounding effects in in vivo experiments. Commonly used antibodies and proteins studied alongside 3H3 (an agonistic anti-4-1BB/CD137 antibody) in the literature include:
In summary, combinatorial approaches with 3H3 in the literature frequently include:
These combinations reflect ongoing efforts to maximize immunotherapeutic efficacy and dissect immune-modulating mechanisms in preclinical models. Clone 3H3 is most frequently cited as a rat monoclonal antibody that acts as a strong agonist for mouse CD137 (4-1BB), a costimulatory receptor important for T-cell activation in immunotherapy, especially in cancer contexts. Key scientific findings from literature citing clone 3H3 include:
Other Contexts: Summary Table: Clone 3H3 (anti-4-1BB/CD137) Key Findings
For most immunology and cancer biology citations, clone 3H3 refers specifically to the anti-mouse CD137 (4-1BB) antibody with the properties summarized above. Dosing regimens of clone 3H3, an agonistic anti-mouse CD137 antibody, vary across mouse models depending on tumor type, mouse strain, and the isotype used, typically ranging from 100–300 μg per mouse per dose, often administered intraperitoneally every 3–7 days.
Summary Table: Dosing of Clone 3H3 in Mouse Models
If you need details for a specific mouse strain, tumor type, or dosing schedule, please specify for a targeted summary. References & CitationsTechnical ProtocolsCertificate of Analysis |
Related Products
Formats Available
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.