Anti-Rotavirus (Clone RV6-25) – Purified No Carrier Protein
Data
- -
- -
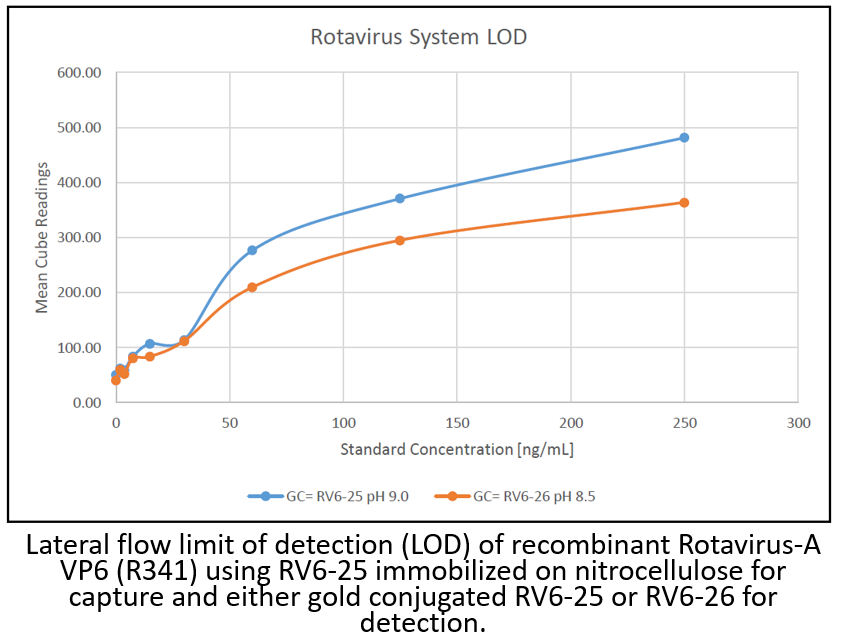
Antibody DetailsProduct DetailsReactive Species Rotavirus ⋅ Virus Expression Host HEK-293 Cells Immunogen Human Mab RV6-25 was sequenced from RV-specific B cells isolated from the blood of healthy adult donors or RV-infected infants or adults5,6. Product Concentration ≥1.0 mg/ml Purity ≥90% monomer by analytical SEC and SDS-Page Formulation This recombinant monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Recombinant antibodies are manufactured in an animal free facility using only in vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Storage and Handling This antibody may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Standard Overnight on Blue Ice. Applications and Recommended Usage? Quality Tested by Leinco Lateral Flow Additional Applications Reported In Literature ? ELISA FC Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity RV6-25 activity is directed against the apical surface of the VP6 head domain. RV6-25 and RV6-26 are encoded by the same heavy chain variable segment6, but unlike RV6-26, RV6-25 is unable to inhibit RV5.
Three-dimensional structural analyses of DLP: Fab complexes show that RV6-25 Fab molecules attach to the tops of VP6 trimers, at one VP6 subunit, with the epitope positioned along the edge and in close contact with the VP6 surface loop5. The RV6-25 Fab protrudes from the VP6 trimers and extends away from the viral surface in a regular pattern depending on the position of the VP6 trimer in the VP6 layer1. Additionally, RV6-25 binds to the apical surface of the DLP VP6 head domain and does not obstruct the transcription pore1. The Fab binds to epitopes on the distinct P, P′, T, T′, or D VP6 trimers. Background Rotaviruses (RV) are double-stranded, non-enveloped, icosahedral RNA viruses in the Reoviridae family1 that cause severe dehydrating diarrhea in infants and children2. RV particles are composed of concentric viral protein (VP) layers3. The triple-layered particle has an inner capsid layer (VP2), an intermediate capsid layer (VP6), and an outer capsid layer (VP7, VP4). The transcriptionally active double-layered particle (DLP) consists of VP2 and VP6. VP6 is the most antigenic RV protein in humans4.
Rotaviruses (RV) are double-stranded, non-enveloped, icosahedral RNA viruses in the Reoviridae family1 that cause severe dehydrating diarrhea in infants and children2. RV particles are composed of concentric viral protein (VP) layers3. The triple-layered particle has an inner capsid layer (VP2), an intermediate capsid layer (VP6), and an outer capsid layer (VP7, VP4). The transcriptionally active double-layered particle (DLP) consists of VP2 and VP6. VP6 is the most antigenic RV protein in humans4. RV6-25 was generated from RV-specific IgD- B cells isolated from the blood of a healthy immune donor5,6 and exhibits moderate affinity5. Mutations that alter residues in the HCDR2 loop exhibit lower affinity than wildtype but result in limited changes in binding kinetics5. RV6-25 and RV6-26 are encoded by the same heavy chain variable segment6, but unlike RV6-26, RV6-25 is unable to inhibit RV5. Three-dimensional structural analyses of DLP:Fab complexes show that RV6-25 Fab molecules attach to the tops of VP6 trimers, at one VP6 subunit, with the epitope positioned along the edge and in close contact with the VP6 surface loop5. The RV6-25 Fab protrudes from the VP6 trimers and extends away from the viral surface in a regular pattern depending on the position of the VP6 trimer in the VP6 layer1. Additionally, RV6-25 binds to the apical surface of the DLP VP6 head domain and does not obstruct the transcription pore1. The Fab binds to epitopes on the distinct P, P′, T, T′, or D VP6 trimers. Combined DXMS and cryo-EM data show that the epitope is within two apical loops of the VP6 structure and that residues 197 to 214 and 308 to 316 in the loops between βB-βC and the short αA helix and βI are directly involved binding. Antigen Distribution VP6 is a viral protein in the intermediate capsid layer of RV. Matched Pair This antibody has been tested in lateral flow assays. The best matched pair is RV6-25 (Capture)/RV6-26 (Detection). Research Area Infectious Disease . Matched Pair . Rotavirus . Viral . IVD Raw Material References & Citations1. Aiyegbo MS, Eli IM, Spiller BW, et al. J Virol. 88(1):469-76. 2014.
2. Bern C, Martines J, de Zoysa I, et al. Bull World Health Organ. 70: 705-714. 1992. 3. Pesavento, JB, Crawford SE, Estes MK, et al. Curr Top Microbiol Immunol. 309: 189-219. 2006. 4. McKinney BA, Kallewaard NL, Crowe JE, et al. Immunome Res 3:8. 2007. 5. Kallewaard NL, McKinney BA, Gu Y, et al. J Immunol. 180(6):3980-9. 2008. 6. Weitkamp JH, Kallewaard NL, Bowen AL, et al. J Immunol. 174(6):3454-60. 2005. 7. Weitkamp JH, Kallewaard N, Kusuhara K, et al. J Immunol Methods. 275:223–237. 2003. Technical ProtocolsCertificate of Analysis |
Related Products
- -
- -
Prod No. | Description |
|---|---|
LT581 | |
R341 |