Anti-Mouse CD4 [Clone GK1.5] — Purified in vivo PLATINUM™ Functional Grade
Anti-Mouse CD4 [Clone GK1.5] — Purified in vivo PLATINUM™ Functional Grade
Product No.: C2838
Clone GK1.5 Target CD4 Formats AvailableView All Product Type Monoclonal Antibody Alternate Names L3T4, T4 Isotype Rat IgG2b κ Applications B , Costim , CyTOF® , Depletion , FA , FC , IHC , in vivo , IP |
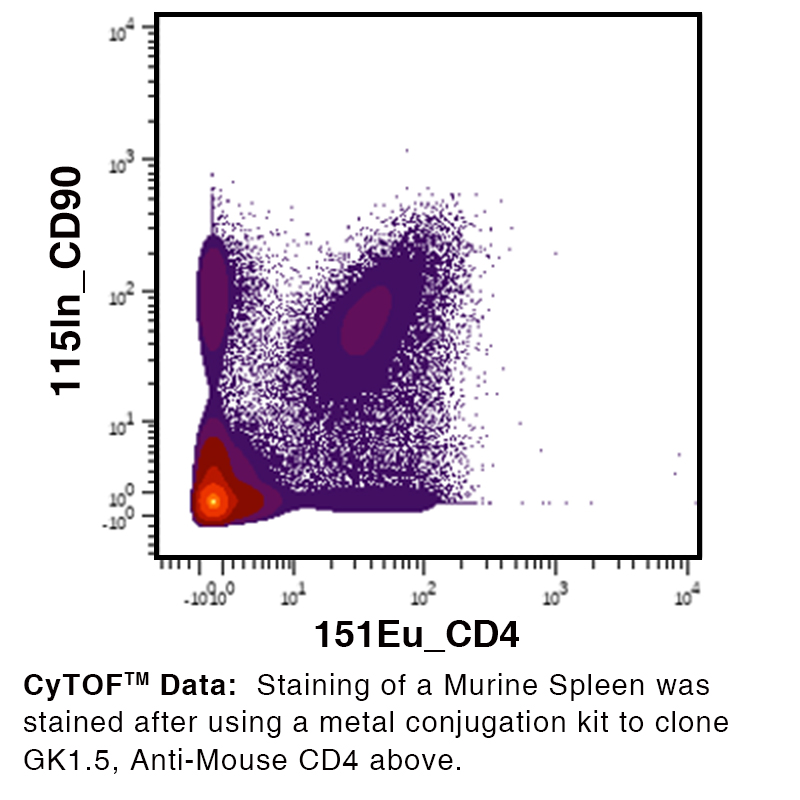
Data
Antibody DetailsProduct DetailsReactive Species Mouse Host Species Rat Recommended Isotype Controls Recommended Isotype Controls Recommended Dilution Buffer Immunogen Mouse CTL clone V4 Product Concentration ≥ 5.0 mg/ml Endotoxin Level <0.5 EU/mg as determined by the LAL method Purity ≥98% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using in vitro cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Pathogen Testing To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s Purified Functional PLATINUM™ antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Next Day 2-8°C RRIDAB_2829596 Applications and Recommended Usage? Quality Tested by Leinco CyTOF® FC The suggested concentration for this GK1.5 antibody for staining cells in flow cytometry is ≤ 1.0 μg per 106 cells in a volume of 100 μl. Titration of the reagent is recommended for optimal performance for each application. Additional Applications Reported In Literature ? B Costim Depletion IHC IP Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Rat Anti-Mouse CD4 (Clone GK1.5) recognizes an epitope on Mouse CD4. This monoclonal antibody was purified using multi-step affinity chromatography methods such as Protein A or G depending on the species and isotype. This antibody was also pathogen tested and third-party certified by IDEXX BioReseach to meet the lowest mycoplasma specification and free of any viral pathogens of concern. Background CD4 (cluster of differentiation 4) is a glycoprotein expressed on the surface of T helper cells, regulatory T cells, monocytes, macrophages, and dendritic cells. CD4 interacts with class II molecules of the major histocompatibility complex (MHC) enhancing the signal for T-cell activation.6 Antigen Distribution The CD4/L3T4 antigen is expressed by the helper/inducer subset of mouse T-cells. The antigen is present on approximately 80% of thymocytes, 20% of spleen cells and 60% of lymph node cells. The expression of L3T4 correlates with class II MHC antigen reactivity on cloned T-cell lines. Ligand/Receptor MHC class II molecule NCBI Gene Bank ID UniProt.org Research Area Immunology Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. The clone GK1.5 is a rat anti-mouse monoclonal antibody specifically targeting the CD4 antigen. It is commonly used for several in vivo applications in mice, including: Common Applications
Technical Applications
These applications highlight the versatility and utility of the GK1.5 antibody in studying immune responses and diseases in mice. When using GK1.5 (anti-mouse CD4 antibody) in research, scientists commonly pair it with antibodies or proteins targeting other immune cell markers to enable detailed immunophenotyping, cell depletion, or mechanistic studies. Frequently used antibodies or proteins in combination with GK1.5 include:
A variety of GK1.5 competitors/blocking antibodies are also referenced in the literature, such as:
In summary, the most commonly used antibodies or proteins with GK1.5 are:
These combinations allow effective immune monitoring, CD4+ T cell depletion studies, and immune subset discrimination in murine models. Key Findings from Clone GK1.5 (Anti-mouse CD4) CitationsDepletion of CD4+ T Cells In Vivo
Blockade of T Cell Activation and Function
Technical and Methodological Studies
Summary Table: Key Applications and Findings
ConclusionClone GK1.5 is a cornerstone tool in immunology research, primarily for depleting and blocking CD4+ T cell function in murine models. Its validated use spans basic research, therapeutic intervention studies, and advanced imaging, consistently demonstrating both specificity and reliability in delineating the roles of CD4+ T cells in health and disease. Dosing Regimens of GK1.5 Across Mouse ModelsDosing regimens for the anti-mouse CD4 antibody clone GK1.5 vary significantly depending on the experimental objectives, mouse strain, route of administration, and desired immunological outcome. There is no universal "one-size-fits-all" protocol, and laboratories must optimize conditions for their specific model and research question. Common Dosing Parameters
Factors Influencing Dosing Regimens
Example Regimens
Optimization and ValidationIt is essential for researchers to perform dose-response experiments to determine the optimal regimen for their specific experimental setup, taking into account mouse strain, age, health, and the primary research objective. Validation by flow cytometry or histological assessment of depletion efficiency is recommended to confirm the adequacy of the chosen regimen. ConclusionGK1.5 dosing regimens in mice are highly context-dependent, with typical doses ranging from 50 to 500 μg, most commonly 200–250 μg intraperitoneally 2–3 times per week for systemic CD4+ T cell depletion. However, the optimal protocol should always be determined empirically for each laboratory’s specific model and goals. References & Citations1.) Ardolino, M. et al. (2018) J Clin Invest. 128(10):4654-4668. PubMed 2.) Schreiber, RD. et al. (2017) Cancer Immunol Res. 5(2):106-117. PubMed 3.) Nicolas, JF. et al. (2002) J Immunol.168(6):3079-87. Article Link 4.) Shin, H. et al. (2018) J Virol. 92(7): e00038-18. PubMed 5.) Chiang, BL. et al. (2001) Immunology. 2001 103(3): 301–309. PubMed 6.) Hendrickson, WA. et al. (1994) Structure 2: 59 7.) Skyberg, J. A. et al. (2020) Infection and Immunity. 88: 5 Journal Link 8.) Hawman DW, et al. (2021) Microorganisms 9(2):279 Journal Link Technical ProtocolsCertificate of Analysis |
Formats Available
Prod No. | Description |
|---|---|
C1840 | |
C211 | |
C214 | |
C212 | |
C213 | |
C338 | |
C1640 | |
C1636 | |
C1333 | |
C1638 | |
C1637 | |
C2838 |
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.