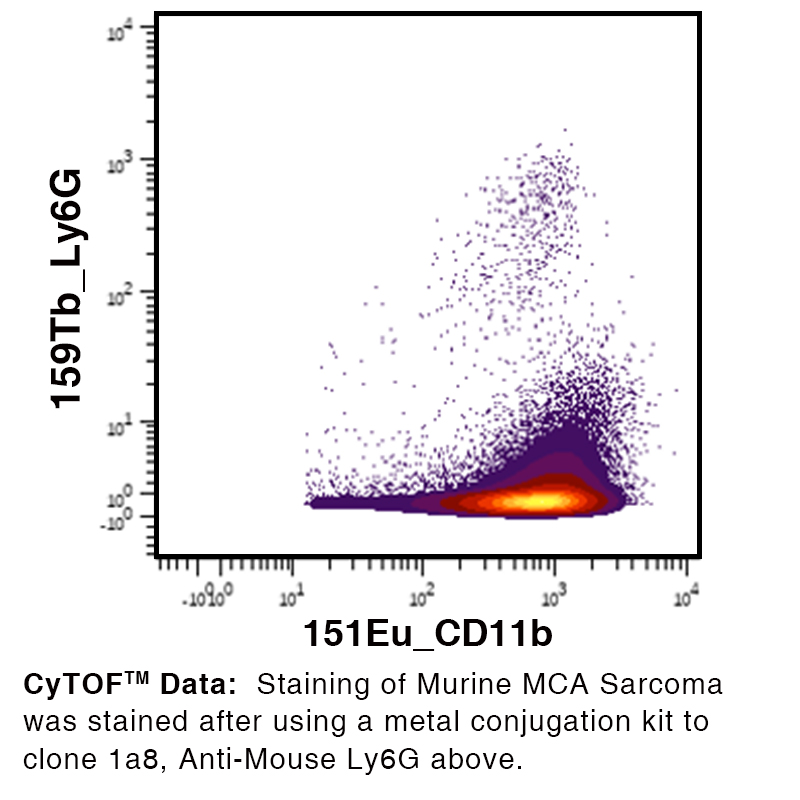
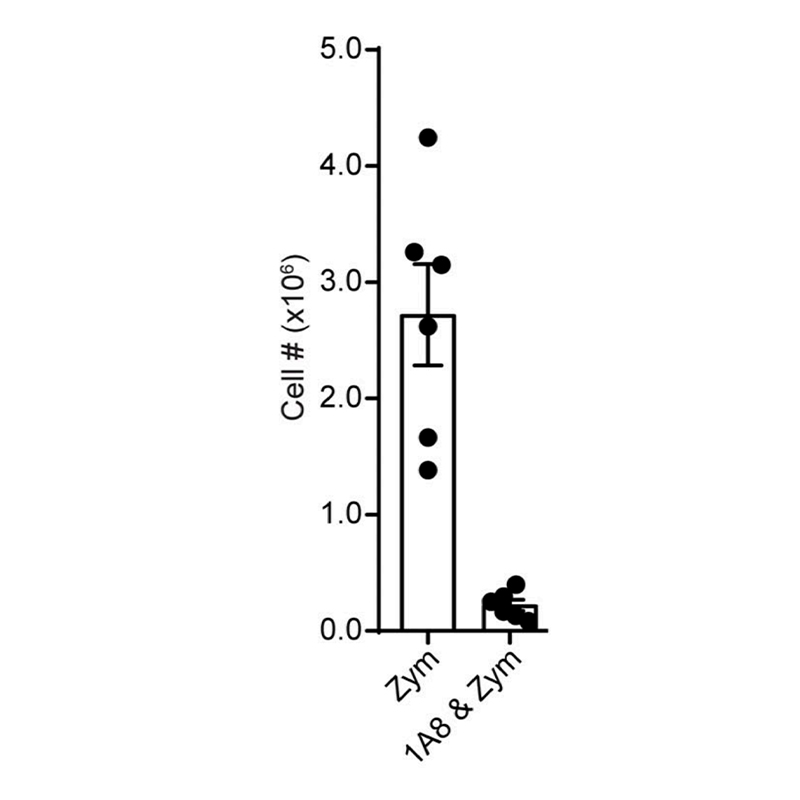
Anti-Mouse Ly-6G [Clone 1A8] — Purified in vivo PLATINUM™ Functional Grade
Anti-Mouse Ly-6G [Clone 1A8] — Purified in vivo PLATINUM™ Functional Grade
Product No.: L305
Clone 1A8 Target Ly-6G Formats AvailableView All Product Type Monoclonal Antibody Alternate Names Lymphocyte Antigen 6 Complex Isotype Rat IgG2a Applications CyTOF® , Depletion , FC , IHC FF , in vivo , PhenoCycler® , WB |
Data
Antibody DetailsProduct DetailsReactive Species Mouse Host Species Rat Recommended Isotype Controls Recommended Dilution Buffer Immunogen Ly-6G transfected EL-4J cell line. Product Concentration ≥ 5.0 mg/ml Endotoxin Level <0.5 EU/mg as determined by the LAL method Purity ≥98% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using in vitro cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Pathogen Testing To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s Purified Functional PLATINUM™ antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Next Day 2-8°C RRIDAB_2893099 Applications and Recommended Usage? Quality Tested by Leinco FC The suggested concentration for this 1A8 antibody for staining cells in flow cytometry is ≤ 0.25 μg per 106 cells in a volume of 100 μl. Titration of the reagent is recommended for optimal performance for each application. Additional Applications Reported In Literature ? CyTOF® Depletion IHC FF WB Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Clone 1A8 recognizes an epitope on mouse Ly6G. Clone 1A8 does not cross react with Ly6C. Background Ly6G antibody, clone 1A8, recognizes lymphocyte antigen 6 complex locus G6D (Ly6G; also called Gr-1), a 21-25 kDa glycosylphosphatidylinositol (GPI)-anchored protein1. Ly6G belongs to the lymphocyte antigen-6 (Ly6)/urokinase-type plasminogen activator receptor (uPAR) superfamily, characterized by a Ly6/uPAR (LU) domain-containing a three-fingered structural motif stabilized by disulfide bonds2. Ly6G is expressed by murine neutrophils regardless of location and activation1,4,5. Eosinophils may also express low levels of Ly6G5. There is no human ortholog for Ly6G; however, a structurally related L76/uPAR protein, CD177 (also known as HNA-2a, NB1, or PRV-1) is expressed in human neutrophils and is implicated in neutropenia6. Although the exact function and ligand of Ly6G remain unknown, Ly6G ligation may impair neutrophil migration to sites of inflammation via a β2-integrin-dependent mechanism7. Antigen Distribution Ly6G is expressed by neutrophils.
PubMed NCBI Gene Bank ID UniProt.org Research Area Immunology . Innate Immunity Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Clone 1A8 is most commonly used in vivo in mice for neutrophil depletion studies. This approach enables precise investigation of neutrophil function in diverse biological contexts, including infectious diseases, cancer, inflammation, wound healing, and autoimmunity. Essential usage details:
Additional in vivo applications:
Other laboratory uses (primarily in vitro or ex vivo, but often ancillary to in vivo work):
Summary Table: Common In Vivo Applications of Clone 1A8 in Mice
Clone 1A8’s specificity and versatility have established it as the gold standard for in vivo neutrophil depletion in mouse immunology and disease modeling research. The antibody 1A8 is most commonly used to target Ly6G for neutrophil depletion in mouse models. In the literature, it is frequently combined with the following antibodies and proteins to delineate immune cell populations or clarify cellular mechanisms:
In practical terms, an example multicolor flow cytometry panel to analyze myeloid and lymphoid subsets might include:
Summary: The most common antibodies or proteins used with 1A8 are those enabling precise leukocyte subset identification—CD11b, Ly6C, CD45, F4/80, alongside lineage markers like CD3e, NK1.1, and B220/CD45R. These combinations are used to characterize, separate, or deplete neutrophils within the broader context of the immune system. Clone 1A8 is widely cited in scientific literature as a highly specific tool for depleting neutrophils in mice, allowing researchers to uncover the precise roles of neutrophils in various immune responses and disease models. Key findings from 1A8-related citations include:
Summary Table—Key Findings from 1A8 Citations
Collectively, literature citations of clone 1A8 have transformed the understanding of neutrophil biology by affording researchers reliable, cell-type specific depletion in mice, thereby clarifying both beneficial and pathogenic roles of neutrophils in immunity and disease. Dosing regimens for clone 1A8 (anti-Ly6G)—used for neutrophil depletion—typically range from 100–250 μg per mouse via intraperitoneal (i.p.) injection every 3 days or three times per week, but these parameters vary significantly depending on mouse strain, age, and disease model. Key factors and variations:
In summary, the optimal dosing regimen of clone 1A8 is highly context-dependent, with mouse strain, age, disease model, and experimental endpoint all requiring careful consideration and experimental validation. The standard starting point is 100–250 μg per mouse, i.p., three times weekly, but dose and schedule should be empirically optimized per model. References & Citations1. Fleming TJ, at al. (1993) J Immunol. 151(5):2399-408 2. Tsetlin VI. (2015) Trends Pharmacol Sci. 36(2):109-23 3. Daley JM, et al. (2008) J Leukoc Biol. 83(1):64-70 4. Lee PY, et al. (2013) J Leukoc Biol. 94(4):585-594 5. Percopo CM, et al. (2017) J Leukoc Biol. 101(1):321-328. 6. Stroncek DF. (2007) Curr Opin Hematol. 14(6):688-93 7. Wang JX, et al. (2012) Blood. 120(7):1489-1498 8. Tzetzo, S. L., Kramer, E. D., Mohammadpour, H., Kim, M., Rosario, S. R., Yu, H., Dolan, M., Oturkar, C. C., Morreale, B., Bogner, P. N., Stablewski, A., Benavides, F., Brackett, C. M., Ebos, J. M., Das, G. M., Opyrchal, M., Nemeth, M. J., Evans, S. S., & Abrams, S. I. (2024). Downregulation of IRF8 in alveolar macrophages by G-CSF promotes metastatic tumor progression. iScience, 109187. https://doi.org/10.1016/j.isci.2024.109187 Technical ProtocolsCertificate of Analysis |
Formats Available
Prod No. | Description |
|---|---|
L286 | |
L279 | |
L283 | |
L282 | |
L285 | |
L281 | |
L287 | |
L288 | |
L289 | |
L500 | |
L280 | |
L284 | |
L305 |
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.