Anti-Mouse CD279 (PD-1) [Clone RMP1-14] — Purified in vivo GOLD™ Functional Grade
Anti-Mouse CD279 (PD-1) [Clone RMP1-14] — Purified in vivo GOLD™ Functional Grade
Product No.: P362
Clone RMP1-14 Target PD-1 Formats AvailableView All Product Type Monoclonal Antibody Alternate Names Programmed Death-1, CD279, PD 1 Isotype Rat IgG2a κ Applications B , FA , FC , IHC , in vivo , WB |
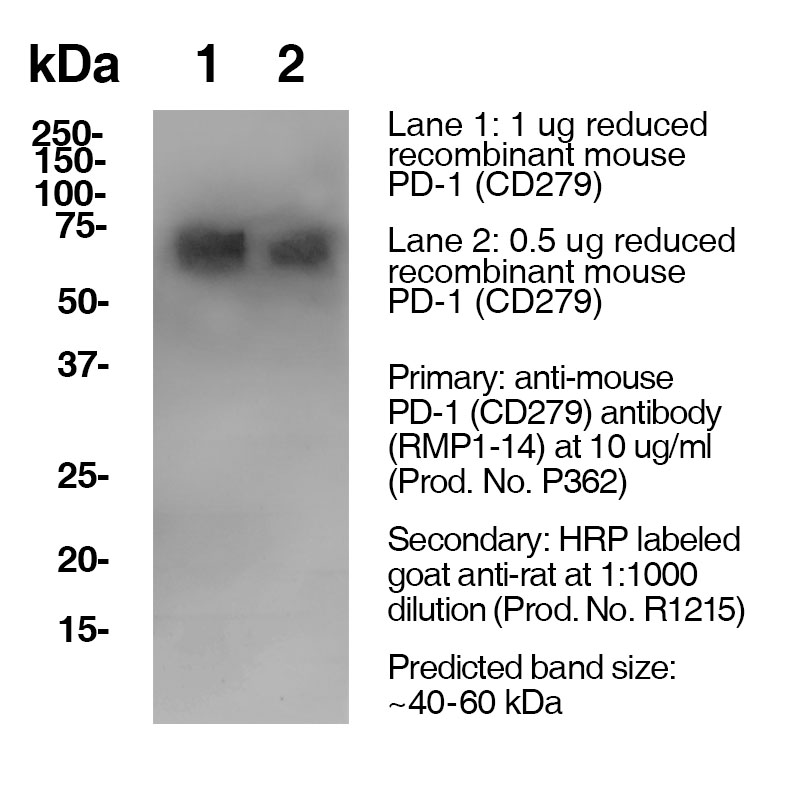
Data
Antibody DetailsProduct DetailsReactive Species Mouse Host Species Rat Recommended Isotype Controls Recommended Isotype Controls Recommended Dilution Buffer Immunogen Mouse PD-1 transfected BHK cells Product Concentration ≥ 5.0 mg/ml Endotoxin Level < 1.0 EU/mg as determined by the LAL method Purity ≥95% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using in vitro cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Next Day 2-8°C RRIDAB_2737557 Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Clone RMP1-14 recognizes an epitope on mouse PD-1. Background PD-1 is a 50-55 kD member of the B7 Ig superfamily. PD-1 is also a member of the extended CD28/CTLA-4 family of T cell regulators and is suspected to play a role in lymphocyte clonal selection and peripheral tolerance. The ligands of PD-1 are PD-L1 and PD-L2, and are also members of the B7 Ig superfamily. PD-1 and its ligands negatively regulate immune responses. PD-L1, or B7-Homolog 1, is a 40 kD type I transmembrane protein that has been reported to costimulate T cell growth and cytokine production. The interaction of PD-1 with its ligand PD-L1 is critical in the inhibition of T cell responses that include T cell proliferation and cytokine production. PD-L1 has increased expression in several cancers. Inhibition of the interaction between PD-1 and PD-L1 can serve as an immune checkpoint blockade by improving T-cell responses In vitro and mediating preclinical antitumor activity. Within the field of checkpoint inhibition, combination therapy using anti-PD1 in conjunction with anti-CTLA4 has significant therapeutic potential for tumor treatments. PD-L2 is a 25 kD type I transmembrane ligand of PD-1. Via PD-1, PD-L2 can serve as a co-inhibitor of T cell functions. Regulation of T cell responses, including enhanced T cell proliferation and cytokine production, can result from mAbs that block the PD-L2 and PD-1 interaction. Antigen Distribution PD-1 is expressed on a subset of CD4-CD8- thymocytes, and on activated T and B cells. Ligand/Receptor PD-L1 (B7-H1), PD-L2 Function Lymphocyte clonal selection, peripheral tolerance PubMed NCBI Gene Bank ID UniProt.org Research Area Apoptosis . Cancer . Cell Biology . Cell Death . Immunology . Inhibitory Molecules . Tumor Suppressors Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Clone RMP1-14 is most commonly used in vivo in mice for studies involving blocking PD-1 signaling, especially in the context of cancer immunotherapy, preclinical anti-tumor efficacy, and immune regulation. Key in vivo applications include:
Practical features:
RMP1-14 is a benchmark antibody for mouse PD-1 blockade studies, valued for its well-established protocols, extensive publication record, and reliability. Commonly used antibodies or proteins employed in combination with RMP1-14 (murine anti-PD-1) in the literature include other anti-PD-1 clones (such as 1A12 and J43), PD-L1/PD-L2 Fc fusion proteins (to assay blocking activity), and immune checkpoint reagents like anti-PD-L1, anti-CTLA-4, and various markers or modulators for T cell studies. Key antibodies and proteins frequently used with RMP1-14:
Summary table:
The most common combinations in the literature are RMP1-14 with 29F.1A12 and J43 for PD-1 functional studies, and RMP1-14 with anti-PD-L1 or anti-CTLA-4 for combination immunotherapeutic models. Using multiple anti-PD-1 clones can help clarify blocking versus agonistic properties, while combining RMP1-14 with checkpoint pathway antibodies (PD-L1, CTLA-4) enables detailed immune interaction studies relevant to cancer immunotherapy. Clone RMP1-14 is a rat IgG2a monoclonal antibody widely used in preclinical research to block the mouse PD-1 immune checkpoint, primarily for in vivo studies of cancer immunotherapy and T cell biology. The key scientific findings from citations involving RMP1-14 are:
In summary, RMP1-14 is an established, well-characterized blocking antibody for mouse PD-1, utilized extensively to model and understand immune checkpoint blockade, optimize dosing, and develop preclinical cancer therapies. It provides consistent, species-specific results with validated protocols, serving as a benchmark and reliable reagent for immuno-oncology studies. Dosing regimens for clone RMP1-14 in mouse models vary based on study objectives, tumor models, mouse strains, immune competency, and administration route, but typical protocols use 200–500 µg per mouse intraperitoneally every 3–4 days. Adjustments are made depending on the specific mouse model, tumor biology, and desired pharmacodynamic effects. Key variations across different mouse models:
Summary Table: Typical RMP1-14 Dosing Regimens in Mice
Important additional notes:
In sum, the 200–500 µg IP every 3–4 days regimen is the most widely used but requires adjustment for specific models or study objectives. Always consult primary data and pilot studies for your particular model. References & Citations1.) Ardolino, M. et al. (2018) J Clin Invest. 128(10):4654-4668. PubMed 2.) Schreiber, RD. et al. (2017) Cancer Immunol Res. 5(2):106-117. 3.) Honjo, T. et al. (1992) EMBO J. 11:3887. 4.) Wurster S. et al. (2020) The Journal of Infectious Diseases 222(6):1989–994 Journal Link 5.) Lo, R. et al. (2021) Cancer Cell 39(10):1375-1387.e6 Journal Link Technical ProtocolsCertificate of Analysis |
Related Products
Prod No. | Description |
|---|---|
S211 | |
R1364 | |
I-1177 | |
C247 | |
F1175 | |
R1214 | |
S571 |
Formats Available
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.
![Anti-Mouse CD279 (PD-1) [Clone RMP1-14] — Purified in vivo GOLD™ Functional Grade, Product vial — Leinco Prod. No. P362](https://www.leinco.com/wp-content/uploads/2025/09/P362-CD279-Clone-RMP1-14-GOLD_Vial-Parent-500x500.jpg)