Anti-Mouse CD279 (PD-1) [Clone 29F.1A12] — Purified in vivo GOLD™ Functional Grade
Anti-Mouse CD279 (PD-1) [Clone 29F.1A12] — Purified in vivo GOLD™ Functional Grade
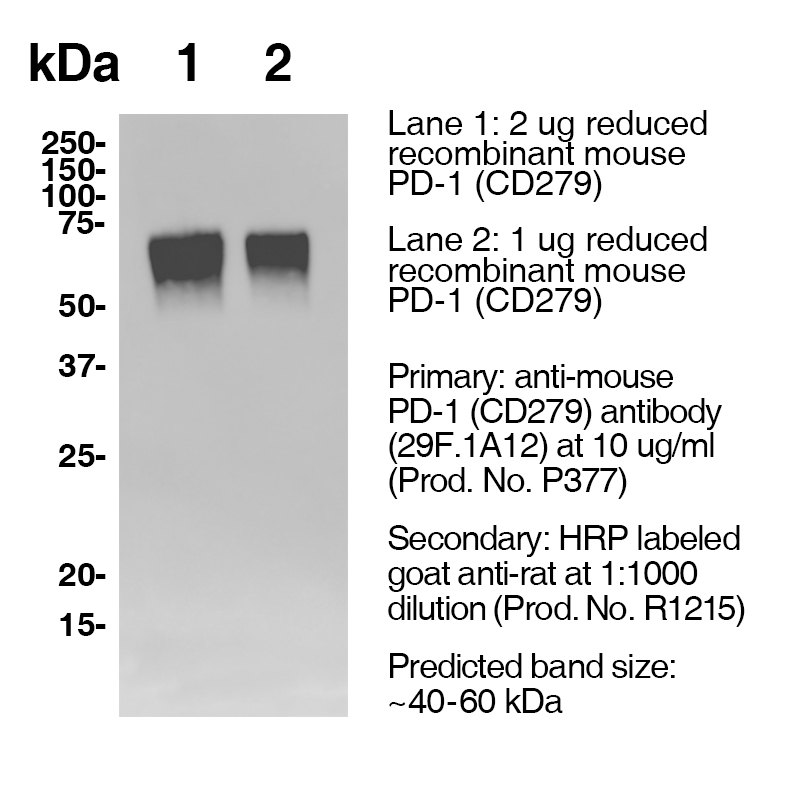
Product No.: P377
Clone 29F.1A12 Target PD-1 Formats AvailableView All Product Type Monoclonal Antibody Alternate Names Programmed Death-1, CD279 Isotype Rat IgG2a Applications B , CyTOF® , FC , IHC FF , in vivo , PhenoCycler® , WB |
Data
Antibody DetailsProduct DetailsReactive Species Mouse Host Species Rat Recommended Isotype Controls Recommended Isotype Controls Recommended Dilution Buffer Immunogen PD-1 cDNA followed by PD-1-Ig fusion protein Product Concentration ≥ 5.0 mg/ml Endotoxin Level < 1.0 EU/mg as determined by the LAL method Purity ≥95% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using in vitro cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Next Day 2-8°C RRIDAB_2737558 Additional Applications Reported In Literature ? CyTOF® Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Clone 29F.1A12 recognizes an epitope on mouse PD-1. Background PD-1 is a 50-55 kD member of the B7 Ig superfamily. PD-1 is also a member of the extended CD28/CTLA-4 family of T cell regulators and is suspected to play a role in lymphocyte clonal selection and peripheral tolerance. The ligands of PD-1 are PD-L1 and PD-L2, and are also members of the B7 Ig superfamily. PD-1 and its ligands negatively regulate immune responses. PD-L1, or B7-Homolog 1, is a 40 kD type I transmembrane protein that has been reported to costimulate T cell growth and cytokine production. The interaction of PD-1 with its ligand PD-L1 is critical in the inhibition of T cell responses that include T cell proliferation and cytokine production. PD-L1 has increased expression in several cancers. Inhibition of the interaction between PD-1 and PD-L1 can serve as an immune checkpoint blockade by improving T-cell responses In vitro and mediating preclinical antitumor activity. Within the field of checkpoint inhibition, combination therapy using anti-PD1 in conjunction with anti-CTLA4 has significant therapeutic potential for tumor treatments. PD-L2 is a 25 kD type I transmembrane ligand of PD-1. Via PD-1, PD-L2 can serve as a co-inhibitor of T cell functions. Regulation of T cell responses, including enhanced T cell proliferation and cytokine production, can result from mAbs that block the PD-L2 and PD-1 interaction. Antigen Distribution PD-1 is expressed on a subset of CD4-CD8- thymocytes, and on activated T and B cells. Ligand/Receptor B7-H1 (PD-L1) and B7-DC (PD-L2) Function Lymphocyte clonal selection, peripheral tolerance NCBI Gene Bank ID UniProt.org Research Area Cancer . Immunology . Inhibitory Molecules Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Clone 29F.1A12 is a rat anti-mouse monoclonal antibody widely used in mice for in vivo immune checkpoint blockade by targeting and blocking the PD-1 receptor, thereby preventing its interaction with its ligands (PD-L1 and PD-L2). Key in vivo applications include:
Additional relevant details:
In summary, 29F.1A12 is most commonly used in live mouse studies for immune checkpoint blockade, chiefly in cancer immunotherapy research, mechanistic T cell studies, and comparative immunotherapy modeling, with proven utility in a broad array of mouse disease models. Commonly Used Antibodies and Proteins with 29F.1A1229F.1A12 is a widely utilized rat anti-mouse PD-1 (CD279) monoclonal antibody, frequently employed in both in vitro and in vivo research to block the PD-1/PD-L1 pathway in mouse models. In the literature, several other antibodies and proteins are commonly paired with 29F.1A12 for comprehensive pathway analysis, combination therapies, or to serve as positive/negative controls. Frequently Used Anti-PD-1 Antibodies
Frequently Used Anti-PD-L1 Antibodies
Functional Proteins and Disease Models
Summary Table
Key Takeaways
The 29F.1A12 monoclonal antibody clone targeting mouse PD-1 (CD279) has generated several important findings across multiple studies, establishing it as a critical tool in immunology and cancer research. Blocking Activity and Ligand InteractionThe most significant characteristic of 29F.1A12 is its exceptional blocking capability. This clone functions as a highly effective blocking antibody that prevents PD-1 from interacting with its ligand PD-L1. The blocking activity is so comprehensive that 29F.1A12 completely prevents PD-1 detection by nearly all other antibody clones when used in competition assays. Among four tested anti-PD-1 clones, 29F.1A12 stands out as the most effective blocking antibody, completely displacing PD-L1-Fc binding at higher concentrations. At concentrations as low as 50 ng/ml, it can reduce PD-L1-Fc binding to PD-1-expressing cells. Binding Specificity and Cell RecognitionStudies have confirmed that 29F.1A12 recognizes surface PD-1 protein on live cells with high specificity. The clone successfully detects PD-1 on live B16-F10 melanoma cells and activated wild-type T-cells. When researchers FACS-purified PD-1+ versus PD-1- subpopulations, they found that Pdcd1 gene expression levels were more than 19-fold enriched in PD-1+ melanoma cell fractions and 6-fold enriched in T-cell isolates. The antibody demonstrates dual recognition patterns when co-stained with another clone (RMP1-30), showing dual positivity by nearly all PD-1 antibody-reactive wild-type melanoma cells (90.8%) and PD-1 overexpressing B16-F10 cells (96.7%). Similarly, overlapping subpopulations of unactivated (57.2%) and activated wild-type T-cells (48.1%) were dually bound by both antibodies. Culture Condition EffectsA notable finding is that 29F.1A12 reactivity varies significantly based on culture conditions. Both 29F.1A12 and RMP1-30 showed more than 3-fold increased reactivity to live wild-type B16-F10 cells in three-dimensional (3D) versus two-dimensional (2D) cultures. This aligns with the observation that the 29F.1A12 PD-1 blocking antibody inhibits B16-F10 melanoma growth in 3D tumor spheroid cultures but not in standard 2D cultures. Cross-Reactivity with Dying CellsAn important caveat identified in the literature is that 29F.1A12 shows some cross-reactivity with dying cells. While the clone maintains PD-1 specificity for live cells, it demonstrates increased reactivity with fixable viability dye-positive (FVD+) cells, indicating reactivity with dead or dying cells. Researchers observed that PD-1 staining with 29F.1A12 uncovered a forward scatter-height (FSC-H) low population predominantly positive for PD-1, which mainly contained dead cells. Functional ApplicationsThe 29F.1A12 antibody has been validated for blocking PD-1 binding to its ligands in vivo, similar to other therapeutic clones like RMP1-14 and J43. This blocking capability has proven valuable in studying cancer immunotherapy, as PD-L1 overexpression in tumors increases resistance to CD8 T cell-mediated lysis, and blocking the PD-1/PD-L1 interaction can transiently arrest tumor growth in mouse models of melanoma. Interestingly, while 29F.1A12 is primarily known as a blocking antibody, weak agonist activity was also detected with this clone, revealing additional functional complexity beyond its primary blocking mechanism. Dosing regimens for clone 29F.1A12 (anti-PD-1 antibody) in mouse models are most commonly 100–200 μg per mouse administered intraperitoneally every 3 days for three doses, but regimens are tailored based on mouse strain, tumor model, and experimental goals. Key dosing variations across mouse models:
Factors influencing dosing variation include:
For most studies, 100–200 μg per mouse, IP, every 3 days for 3 doses remains the reference regimen, but deviations are common based on experimental needs and mouse model specifics. References & Citations1.) Ardolino, M. et al. (2018) J Clin Invest. 128(10):4654-4668. PubMed 2.) Schreiber, RD. et al. (2017) Cancer Immunol Res. 5(2):106-117. 3.) Honjo, T. et al. (1992) EMBO J. 11:3887. 4.) Wurster S. et al. (2020) The Journal of Infectious Diseases 222(6):1989–994 Journal Link 5.) Lo, R. et al. (2021) Cancer Cell 39(10):1375-1387.e6 Journal Link Technical ProtocolsCertificate of Analysis |
Related Products
Prod No. | Description |
|---|---|
S211 | |
R1364 | |
I-1177 | |
C247 | |
F1175 | |
R1214 | |
S571 |
Formats Available
Prod No. | Description |
|---|---|
P377 | |
P383 | |
P384 | |
P501 | |
P378 |
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.
![Anti-Mouse CD279 (PD-1) [Clone 29F.1A12] — Purified in vivo GOLD™ Functional Grade, parent vial — Leinco Prod. No. P377](https://www.leinco.com/wp-content/uploads/2025/09/P377-Anti-Mouse-CD279-PD-1-Clone-29F.1A12-GOLD-Parent-Vial-500x500.jpg)