Anti-SARS-CoV-2 Nucleocapsid (N) (Clone NP2-F6) – Purified No Carrier Protein
Anti-SARS-CoV-2 Nucleocapsid (N) (Clone NP2-F6) – Purified No Carrier Protein
Product No.: LT7025
- -
- -
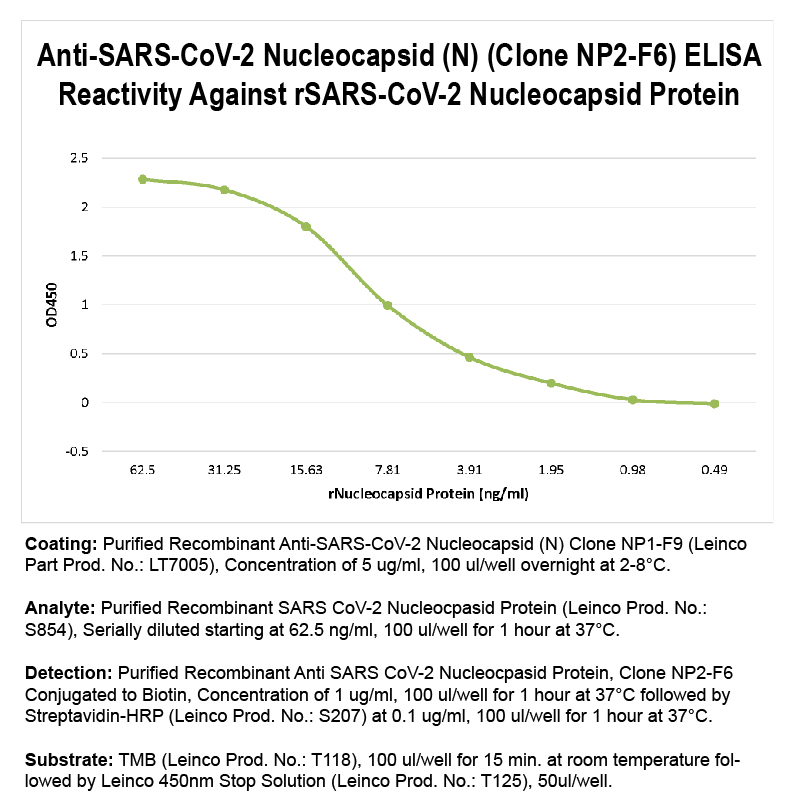
Product No.LT7025 Clone NP2-F6 Target SARS-CoV-2 Nucleocapsid (N) Product Type Recombinant Monoclonal Antibody Alternate Names COV2-NP2-F6, SARS-CoV-2 Nucleocapsid, SARS-CoV-2 Nucleoprotein, Protein N, SARS-CoV N Protein Isotype Human IgG1 Applications ELISA |
Data
- -
- -
Antibody DetailsProduct DetailsReactive Species SARS-CoV-2 ⋅ Virus Host Species Mouse Expression Host HEK-293 Cells Immunogen SARS-CoV-2 Nucleocapsid (N) Protein Product Concentration ≥1.0 mg/ml Purity ≥90% monomer by analytical SEC and SDS-Page Formulation This recombinant monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Recombinant antibodies are manufactured in an animal free facility using only in vitro protein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Storage and Handling This antibody may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Ships Overnight on Blue Ice RRIDAB_2892939 Applications and Recommended Usage? Quality Tested by Leinco ELISA Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Anti-SARS-CoV-2 Nucleocapsid, clone NP2-F6, specifically targets an epitope on the SARS-CoV-2 nucleocapsid protein. Background Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 belongs to the Coronaviridae family, and its single-stranded, positive-sense RNA genome shares 79.6% identity with SARS-CoV1. The spike (S), envelope (E), membrane (M), and nucleocapsid proteins (N) are four essential structural proteins of SARS-CoV-22. The 46 kDa N protein is highly conserved and shares 90% homology with SARS-CoV3. Similar to SARS-CoV, SARS-CoV-2 has an N-terminal (NTD) and C-terminal domain (CTD), linked by a linker region. The NTD binds to RNA, while the CTD self-oligomerizes4,5, aiding viral genome packaging into a helical ribonucleoprotein complex6. The N protein also participates in viral transcription, replication, and modulation of cell signaling pathways7,8. Some vaccine and diagnostic assays9 have focused on the N protein as it is highly expressed during infection and activates antibodies3,10 and memory T cells11,12, found in convalescent sera. The N-protein also evades the innate immune system by inhibiting RNAi13, identifying it as a potential therapeutic target. Antigen Distribution The nucleocapsid protein is expressed in the internal nucleocapsid of SARS-CoV-2. NCBI Gene Bank ID Research Area COVID-19 . Infectious Disease . Seasonal and Respiratory Infections . Viral . IVD Raw Material References & Citations1. Zhou, P., Yang, X., Wang, X. et al. Nature 579, 270–273. 2020. 2. Wu, F., Zhao, S., Yu, B. et al. Nature 579, 265–269. 2020. 3. Guo L., Ren L., Yang S., et al. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America. 2020. 4. Kang S, Yang M, Hong Z, et al. Acta Pharm Sin B. 10.1016/j.apsb.2020.04.009. 2020. 5. Chang CK, Sue SC, Yu TH, et al. J Biomed Sci. 13(1):59-72. 2006. 6. Hsieh PK, Chang SC, Huang CC, et al. J Virol. 79(22):13848-13855. 2005. 7. Surjit M, Lal SK. Infect Genet Evol. 8(4):397-405. 2008. 8. Hurst KR, Ye R, Goebel SJ, Jayaraman P, Masters PS. J Virol. 84(19):10276-10288. 2010. 9. Liu L, Liu W, Zheng Y, et al. Microbes Infect. 22(4-5):206-211. 2020. 10. To K.K., Tsang O.T., Leung W.S., et al. Lancet Infect. Dis. 2020. 11. Grifoni A., Weiskopf D., Ramirez S.I., et al. Cell. 2020. 12. Ni L, Ye F, Cheng ML, et al. Immunity. 52(6):971-977.e3. 2020. 13. Mu J, Xu J, Zhang L, et al. Sci China Life Sci. 1-4. 2020. Technical ProtocolsCertificate of Analysis |
Related Products
- -
- -
Prod No. | Description |
|---|---|
LT1900 | |
LT2000 | |
S854 | |
LT3000 | |
LT4000 | |
LT5000 | |
LT6000 |