Anti-SARS-CoV-2 Nucleocapsid (N) (Clone NP1-E9) – HRP
Anti-SARS-CoV-2 Nucleocapsid (N) (Clone NP1-E9) – HRP
Product No.: LT7017
- -
- -
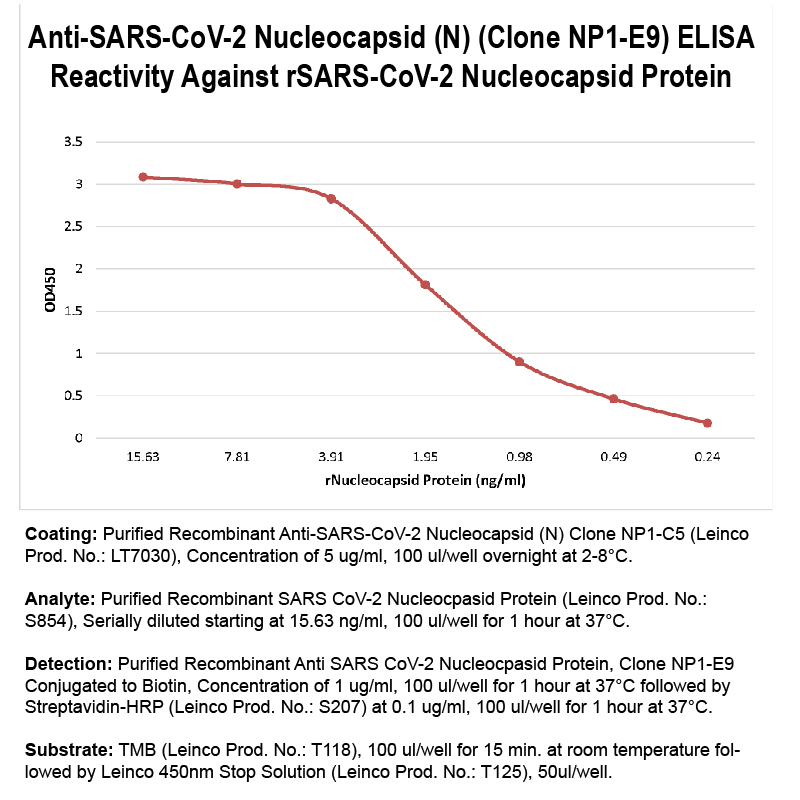
Product No.LT7017 Clone NP1-E9 Target SARS-CoV-2 Nucleocapsid (N) Product Type Recombinant Monoclonal Antibody Alternate Names COV2-NP1-E9, SARS-CoV-2 Nucleocapsid, SARS-CoV-2 Nucleoprotein, Protein N, SARS-CoV N Protein Isotype Human IgG1 Applications ELISA |
Data
- -
- -
Antibody DetailsProduct DetailsReactive Species SARS-CoV-2 ⋅ Virus Host Species Mouse Expression Host HEK-293 Cells Immunogen SARS-CoV-2 Nucleocapsid (N) Protein Product Concentration 0.5 mg/ml Formulation This HRP-conjugated antibody is formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4, 1% BSA. (Warning: Use of sodium azide as a preservative will inhibit the enzyme activity of horseradish peroxidase) Storage and Handling This horseradish peroxidase conjugated monoclonal antibody is stable when stored at 2-8°C. Do not freeze. Country of Origin USA Shipping Ships Overnight on Blue Ice RRIDAB_2893985 Applications and Recommended Usage? Quality Tested by Leinco ELISA Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Anti-SARS-CoV-2 Nucleocapsid, clone NP1-E9, specifically targets an epitope on the SARS-CoV-2 nucleocapsid protein. Futhermore, it is reported to bind to the oligomerization domain of the N protein. Background Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), is an enveloped, single-stranded, positive-sense RNA virus that belongs to the Coronaviridae family 1. The SARS-CoV-2 genome, which shares 79.6% identity with SARS-CoV, encodes four essential structural proteins: the spike (S), envelope (E), membrane (M), and nucleocapsid protein (N) 2. The N protein is 46 kDa and consists of two highly conserved structural domains, the N-terminal domain (NTD) and C-terminal domain (CTD), connected by a linker region. The NTD and CTD are involved in RNA binding and self-oligomerization, respectively 3, 4. The primary function of the N protein is to bind to and package the viral RNA genome into a helical ribonucleoprotein complex 5. The N protein is also involved in other critical steps of the viral life cycle, including transcription, replication, and modulating infected cell signaling pathways 6, 7. The N protein is abundantly expressed during infection and is highly conserved, sharing 90% amino acid homology with the SARS-CoV N protein 8. It is also immunogenic, and antibodies 8,9 and memory T cells 10, 11 targeting the N protein are present in the sera of convalescent COVID-19 patients, identifying the N protein as a suitable candidate for vaccine development and diagnostic assays. Diagnostic assays based on the N protein effectively detect antibodies in the sera of patients infected with SARS-CoV-2 12. The N protein also contributes to immune evasion by antagonizing antiviral RNAi 13, suggesting its potential value as a targeted therapeutic. Antigen Distribution The nucleocapsid protein is expressed in the internal nucleocapsid of SARS-CoV-2. NCBI Gene Bank ID Research Area COVID-19 . Infectious Disease . Seasonal and Respiratory Infections . Viral . IVD Raw Material References & Citations1. Zhou, P., Yang, X., Wang, X. et al. Nature 579, 270–273. 2020. 2. Wu, F., Zhao, S., Yu, B. et al. Nature 579, 265–269. 2020. 3. Kang S, Yang M, Hong Z, et al. Acta Pharm Sin B. 10.1016/j.apsb.2020.04.009. 2020. 4. Chang CK, Sue SC, Yu TH, et al. J Biomed Sci. 13(1):59-72. 2006. 5. Hsieh PK, Chang SC, Huang CC, et al. J Virol. 79(22):13848-13855. 2005. 6. Surjit M, Lal SK. Infect Genet Evol. 8(4):397-405. 2008. 7. Hurst KR, Ye R, Goebel SJ, Jayaraman P, Masters PS. J Virol. 84(19):10276-10288. 2010. 8. Guo L., Ren L., Yang S., et al. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America. 2020. 9. To K.K., Tsang O.T., Leung W.S., et al. Lancet Infect. Dis. 2020. 11. Ni L, Ye F, Cheng ML, et al. Immunity. 52(6):971-977.e3. 2020. 12. Liu L, Liu W, Zheng Y, et al. Microbes Infect. 22(4-5):206-211. 2020. 13. Mu J, Xu J, Zhang L, et al. Sci China Life Sci. 1-4. 2020. Technical ProtocolsCertificate of Analysis |
Related Products
- -
- -
Prod No. | Description |
|---|---|
LT1900 | |
LT2000 | |
S854 | |
LT3000 | |
LT4000 | |
LT5000 | |
LT6000 |