Recombinant Human GM-CSF
Data
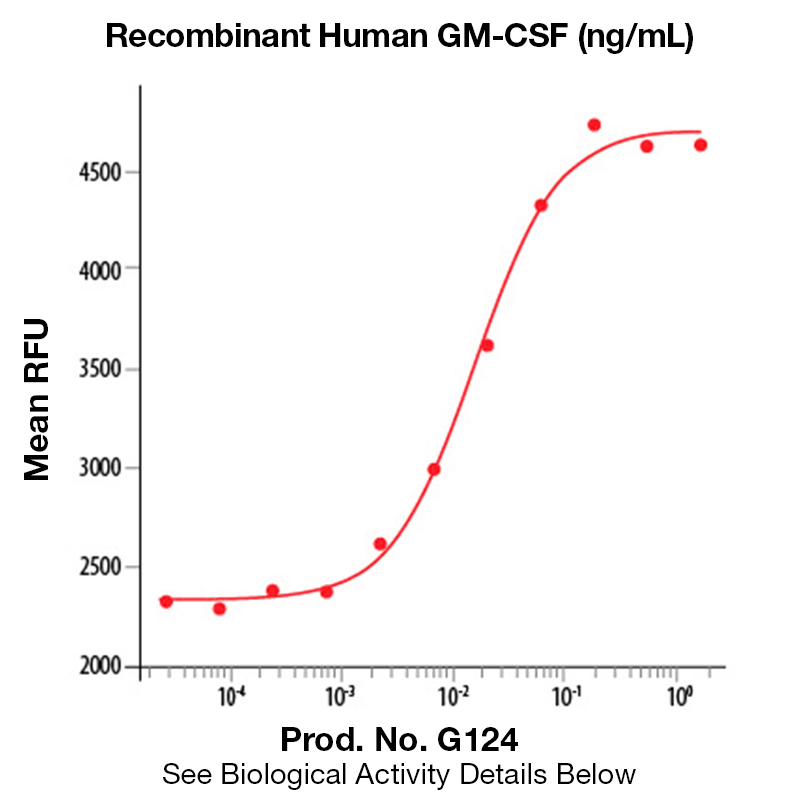
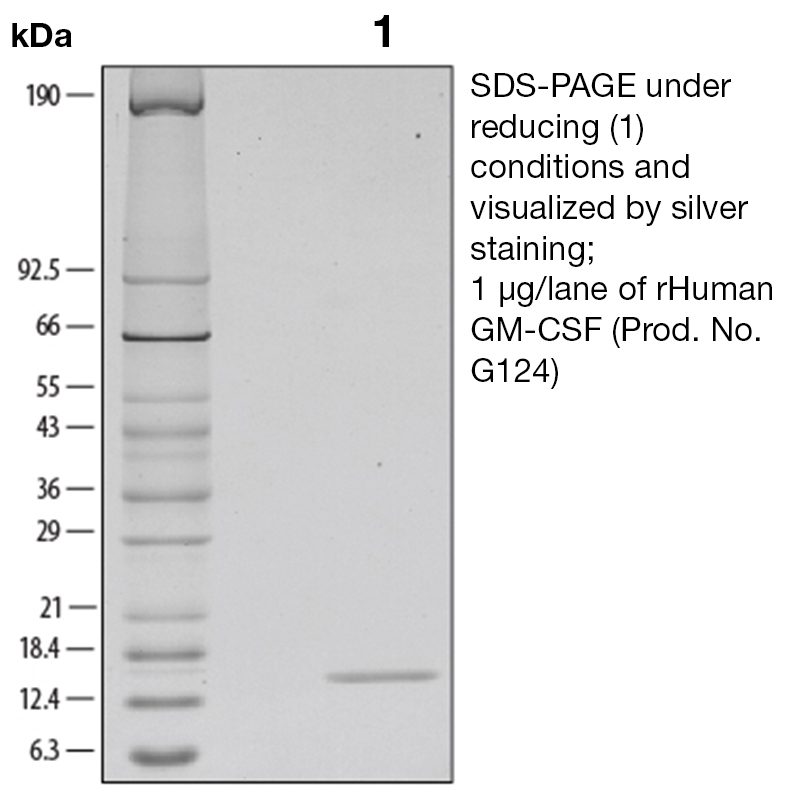
BackgroundGranulocyte-Macrophage Colony Stimulating Factor is a 22 kD, pleiotropic cytokine that is a white blood cell growth factor. It controls the production and function of blood cells by stimulating stem cells to produce granulocytes and monocytes. GM-CSF differs from G-CSF in that it affects more cell types including macrophages and eosinophils. Moreover, GM-CSF is part of the immune/inflammatory cascade, a process crucial for fighting infection. Interestingly, GM-CSF expression may have pathological implications. Autocrine expression of GM-CSF in myeloid leukemia cells is suspected to play a role in neoplasia, the formation of a new and abnormal growth of tissue. Additionally, GM-CSF expression has also been documented in certain solid tumors. There have also been reports of GM-CSF in synovial fluid from patients with arthritis suggesting that GM-CSF may play a role in tissue damage associated with the inflammatory process. Blocking GM-CSF is thought to have therapeutic potential by reducing inflammation. Some drugs are currently being developed to block GM-CSF. Protein DetailsPurity >97% by SDS-PAGE and analyzed by silver stain. Endotoxin Level <1.0 EU/µg as determined by the LAL method Biological Activity The biological activity of Human GM-CSF was determined by a cell proliferation assay using the factor-dependent cell line, TF-1 (Kitamura, T. et al., 1989, J. Cell Physiol. 140:323 - 343). The expected ED<sub>50</sub> for this effect is typically 6 - 30 pg/ml. Protein Accession No. CAA26822 Amino Acid Sequence apa rspspstqpw ehvnaiqear rllnlsrdta aemnetvevi semfdlqept clqtrlelyk qglrgsltkl kgpltmmash ykqhcpptpe tscatqiitf esfkenlkdf llvipfdcwe pvqe
N-terminal Sequence Analysis Ala18 State of Matter Lyophilized Predicted Molecular Mass The predicted molecular weight of Recombinant Human GM-CSF is Mr 14 kDa. Additionally, the actual molecular weight as observed by migration on SDS-PAGE is 14 kDa (reducing conditions). Predicted Molecular Mass 14 Formulation This recombinant protein solution was 0.2 µm filtered and formulated in modified Dulbecco’s phosphate buffered saline (1X PBS) pH 7.2 – 7.3 with no calcium, magnesium, or preservatives present. Storage and Stability This protein is stable for up to twelve months when stored at -20°C to -70°C. After thawing and addition of a carrier protein, this protein may be stored at 2°C to 8°C for one month or for long-term storage, aliquot and store at -20°C to -70°C in a manual defrost freezer. Avoid Repeated Freeze Thaw Cycles. See Product Insert for exact lot specific storage instructions. Country of Origin USA Shipping Next Day Ambient NCBI Gene Bank Leinco Protein AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor (rHuGM-CSF) is a highly valuable tool for research applications due to its broad immunomodulatory and hematopoietic effects. Here are the key reasons to use rHuGM-CSF in your research: 1. Enhances Immune Cell Function
2. Promotes Hematopoietic Recovery
3. Supports Cancer Immunotherapy Research
4. Modulates Inflammatory and Autoimmune Responses
5. Improves Host Defense and Infection Models
6. High Biological Activity and Reproducibility
7. Broad Research Applications
In summary, rHuGM-CSF is a powerful and versatile cytokine for research applications, offering robust effects on immune cell function, hematopoiesis, and disease modeling. Its use can provide critical insights into immune regulation, cancer immunotherapy, and the pathogenesis of various diseases. Yes, recombinant human GM-CSF can be used as a standard for quantification and calibration in ELISA assays. This is a well-established practice in research applications. Suitability as ELISA StandardsRecombinant human GM-CSF proteins are specifically designed and validated for use as ELISA standards. The recombinant protein is typically produced in E. coli expression systems and is calibrated against recognized international reference standards, such as the WHO International Standard (NIBSC code: 88/646). This calibration ensures traceability and consistency across different assays and laboratories. Key Considerations for UseCarrier Protein Requirements When using recombinant GM-CSF as an ELISA standard, carrier protein concentrations of 5–10 mg/mL are recommended. This is particularly important for maintaining protein stability and preventing non-specific binding to assay surfaces. Animal-free formulations are also available if your research requires non-animal-derived reagents. Validation with Natural GM-CSF An important advantage of recombinant standards is their compatibility with natural GM-CSF detection. Studies have demonstrated that results obtained using natural human GM-CSF show linear curves that are parallel to standard curves generated using recombinant GM-CSF standards. This indicates that recombinant standards can reliably determine relative mass values for natural GM-CSF, making them suitable for quantifying both recombinant and endogenous GM-CSF in biological samples. Typical Performance Characteristics Recombinant GM-CSF standards enable ELISA assays to achieve sensitivity levels typically less than 10 pg/mL, with standard curve ranges around 10–250 pg/mL, though some assays achieve wider detection ranges. The specific activity of recombinant human GM-CSF is typically greater than 1.0 × 10⁷ IU/mg, providing reliable quantification across physiologically relevant concentration ranges. Recombinant human GM-CSF has been validated for a diverse range of applications spanning both research and clinical settings. Research and Analytical ApplicationsIn laboratory research, recombinant GM-CSF has been validated for Functional Assay, ELISA, Western Blot, Immunohistochemistry, Blocking Assay, and Immunoprecipitation. These applications enable researchers to study the protein's biological activity, detect its presence in samples, visualize its localization in tissues, and investigate its interactions with other molecules. Cell Culture and Differentiation StudiesThe protein is extensively used in cell culture and differentiation studies. Specifically, it has been validated for dendritic cell generation and differentiation, as well as for culturing specialized cell types such as microglia derived from human induced pluripotent stem cells. These applications leverage GM-CSF's well-characterized ability to promote proliferation and differentiation of hematopoietic progenitors and the generation of neutrophils, eosinophils, and macrophages. Cell Proliferation StudiesRecombinant GM-CSF has been validated for cell proliferation assays, allowing researchers to quantify its mitogenic effects on target cell populations. Therapeutic and Clinical ApplicationsBeyond research applications, recombinant GM-CSF has demonstrated clinical efficacy in multiple therapeutic contexts. These include myeloid reconstitution after bone marrow and blood transplantation, acceleration of neutrophil recovery following chemotherapy for acute myeloid leukemia, and treatment of severe myelosuppression. Emerging applications under investigation include its use as a vaccine adjuvant, immunotherapy for malignancies, and treatment of infections in immunosuppressed populations. Additionally, research has validated its use in wound healing and burn treatment, as well as potential applications in neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease. To reconstitute and prepare Recombinant Human GM-CSF protein for cell culture experiments, briefly centrifuge the lyophilized vial before opening, then add sterile water or buffer to achieve a concentration between 0.1–1.0 mg/mL. Gently mix without vortexing, and if needed, add carrier protein such as 0.1% human or bovine serum albumin (HSA/BSA) to improve stability and prevent adsorption to surfaces. Step-by-step protocol:
Additional notes:
Summary Table:
This protocol ensures optimal activity and stability of recombinant GM-CSF for cell culture applications. References & Citations1. Parker, MW. et al. (2008) Cell 134:496 2. Esnault, S. et al. (2002) Arch. Immunol. Ther. Exp. (Warsz.) 50:121 3. Yong, KL. et al. (1993) J. Immunol. 150:2449 4. Whitsett, JA. et al. (2002) Annu. Rev. Physiol. 64:775 5. Armitage, JO. et al. (1992) Sem. Hematol. 29:14 6. Eksioglu, EA. et al. (2007) Exp. Hematol. 35:1163 Certificate of AnalysisIMPORTANT Use lot specific datasheet for all technical information pertaining to this recombinant protein. |
Related Products
Prod No. | Description |
|---|---|
G665 | |
G675 | |
G596 | |
G128 | |
G117 | |
G672 | |
G681 | |
G119 | |
G124 | |
G142 | |
G143 | |
G691 |
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.