Anti-Mouse/Human CD273 (PD-L2) [Clone 3.2.B8] — Purified in vivo PLATINUM™ Functional Grade
Anti-Mouse/Human CD273 (PD-L2) [Clone 3.2.B8] — Purified in vivo PLATINUM™ Functional Grade
Product No.: P679
Clone 3.2.B8 Target PD-L2 Formats AvailableView All Product Type Monoclonal Antibody Alternate Names B7-DC, CD273, PDL2, B7DC, Clone 3.2 Isotype Mouse IgG1 Applications B , FC , in vivo , WB |
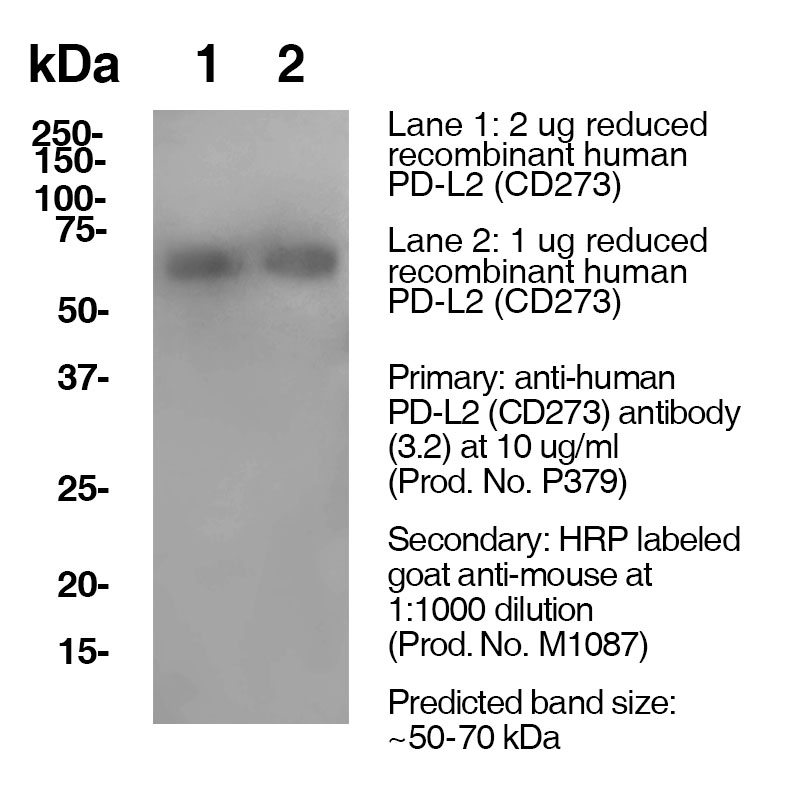
Data
Antibody DetailsProduct DetailsReactive Species Human ⋅ Mouse Host Species Mouse Recommended Isotype Controls Recommended Isotype Controls Recommended Dilution Buffer Immunogen Made in PD-L2 knockout mouse fusion partner X63-Ag8.653 myeloma cells Product Concentration ≥ 5.0 mg/ml Endotoxin Level <0.5 EU/mg as determined by the LAL method Purity ≥98% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using in vitro cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Pathogen Testing To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s Purified Functional PLATINUM™ antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Next Day 2-8°C RRIDAB_2831658 Applications and Recommended Usage? Quality Tested by Leinco Western Blot: For Western blot analysis at a concentration of 1.0-2.0 µg/ml when used in conjunction with compatible secondary reagents Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity Clone 3.2.B8 recognizes an epitope on mouse/human PD-L2 and has been shown to bind to both mouse and human PD-L2 equally well Background PD-1 is a 50-55 kD member of the B7 Ig superfamily. PD-1 is also a member of the extended CD28/CTLA-4 family of T cell regulators and is suspected to play a role in lymphocyte clonal selection and peripheral tolerance. The ligands of PD-1 are PD-L1 and PD-L2, and are also members of the B7 Ig superfamily. PD-1 and its ligands negatively regulate immune responses. PD-L1, or B7-Homolog 1, is a 40 kD type I transmembrane protein that has been reported to costimulate T cell growth and cytokine production. The interaction of PD-1 with its ligand PD-L1 is critical in the inhibition of T cell responses that include T cell proliferation and cytokine production. PD-L1 has increased expression in several cancers. Inhibition of the interaction between PD-1 and PD-L1 can serve as an immune checkpoint blockade by improving T-cell responses In vitro and mediating preclinical antitumor activity. Within the field of checkpoint inhibition, combination therapy using anti-PD1 in conjunction with anti-CTLA4 has significant therapeutic potential for tumor treatments. PD-L2 is a 25 kD type I transmembrane ligand of PD-1. Via PD-1, PD-L2 can serve as a co-inhibitor of T cell functions. Regulation of T cell responses, including enhanced T cell proliferation and cytokine production, can result from mAbs that block the PD-L2 and PD-1 interaction. Antigen Distribution PD-L2 is expressed on dendritic cells, liver, few transformed cell lines, and a subset of macrophages. Ligand/Receptor PD-1 Function Binds to PD-1 and alternative receptor NCBI Gene Bank ID UniProt.org Research Area Costimulatory Molecules . Immunology Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. Clone 3.2.B8 is a monoclonal antibody that targets PD-L2 (CD273) and is widely used in mice for in vivo immune checkpoint blockade studies, particularly to modulate T cell responses by inhibiting the PD-L2/PD-1 interaction. Key in vivo applications include:
Additional details:
Overall, clone 3.2.B8 is a well-characterized in vivo tool for probing the function of PD-L2 in mouse models of immunity and immunotherapy. Commonly used antibodies or proteins studied with clone 3.2.B8 (anti-mouse PD-L2/CD273) in the literature include:
These combinations are common in studies investigating immune checkpoint regulation, T cell activation, and immune cell phenotyping. They are especially prevalent in immuno-oncology and autoimmunity research contexts, as blocking the PD-1/PD-L2 axis often requires co-detection of these critical immune markers to fully characterize the cellular and functional effects of PD-L2 blockade. In checkpoint inhibition research, anti-PD1 and anti-CTLA4 antibodies are also commonly used alongside 3.2.B8 to study synergistic or combinatorial therapeutic effects. However, 3.2.B8 is most often combined specifically with phenotyping and additional inhibitory/stimulatory checkpoint markers to map immune modulation in vivo and in vitro. Clone 3.2.B8 is a monoclonal antibody widely cited in scientific literature as a highly specific tool for detecting PD-L2 (CD273) on both mouse and human cells. Key findings from its citations include:
In summary, scientific literature cites clone 3.2.B8 as a critical reagent for mechanistic studies and therapeutic investigations involving PD-L2 in immune regulation, especially in mouse models of immunity and cancer, but not in infectious disease contexts. Dosing Regimens of Clone 3.2.B8 in Mouse ModelsNo Standardized or Model-Specific Dosing for Clone 3.2.B8 The search results specifically highlight that there is no direct, detailed, or standardized dosing information for clone 3.2.B8 (anti-mouse CD273/PD-L2) in mouse models. The product listing and available scientific literature do not provide model-specific or disease-specific dosing regimens for this clone. This contrasts with other widely used antibodies (e.g., anti-CTLA-4, anti-NK1.1), for which standardized dose ranges and schedules are well-documented. General Guidance and RecommendationsAdapting from Similar Antibodies When direct data are unavailable, researchers are advised to adopt dosing regimens from antibodies targeting similar pathways (e.g., other checkpoint inhibitors like anti-PD-1 or anti-CTLA-4), and to adjust for the specific biology of the mouse model being used. For most immune checkpoint antibodies in mice, standard doses typically range from 5–300 μg per mouse, administered via intraperitoneal (i.p.) or intravenous (i.v.) injection. Dosing for Related Immune Checkpoint Antibodies Critical Steps for Protocol Development
Summary Table: Clone 3.2.B8 Dosing in Mouse Models
ConclusionThere is no established, model-specific dosing regimen for clone 3.2.B8 in mouse models. Researchers must base initial dosing on general guidelines for immune checkpoint antibodies, then empirically determine the optimal regimen for their specific experimental context through careful validation. Always consult recent literature and, if possible, contact the antibody provider or collaborating labs for unpublished data or recommendations. References & Citations1.) Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, Sharpe AH, Umetsu
DT, Dekruyff RH. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-
dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010; 3:81-
91. PMCID: PMC2845714 2.) . Xiao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, Hua P, Duke-Cohan JS, Umetsu DT, Sharpe AH, DeKruyff RH*, Freeman GJ* (* indicates co-senior authors). RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014; 211:943-59. PMCID: PMC4010901. Technical ProtocolsCertificate of Analysis |
Related Products
Prod No. | Description |
|---|---|
S211 | |
R1364 | |
I-536 | |
M1188 | |
C247 | |
F1175 | |
S571 |
Formats Available
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.