Anti-Mouse CD223 (LAG-3) [Clone C9B7W] — Purified in vivo GOLD™ Functional Grade
Anti-Mouse CD223 (LAG-3) [Clone C9B7W] — Purified in vivo GOLD™ Functional Grade
Product No.: L306
Clone C9B7W Target CD223 Formats AvailableView All Product Type Monoclonal Antibody Alternate Names CD223, LAG3 Isotype Rat IgG1 κ Applications B , FA , FC , in vivo , IP , WB |
Data
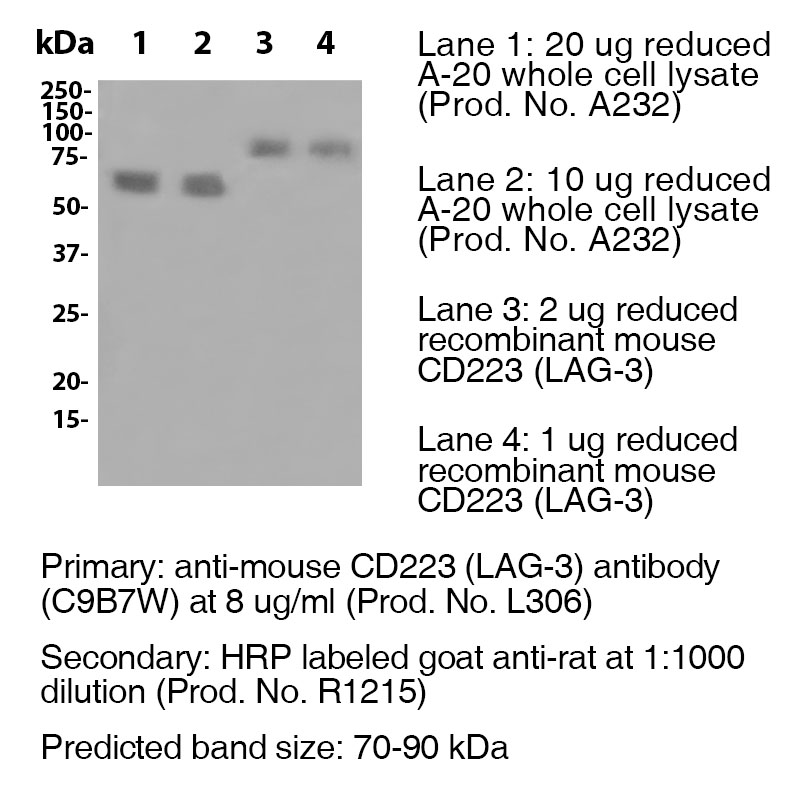
Antibody DetailsProduct DetailsReactive Species Mouse Host Species Rat Recommended Isotype Controls Recommended Isotype Controls Recommended Dilution Buffer Immunogen Murine CD223-Ig fusion protein Product Concentration ≥ 5.0 mg/ml Endotoxin Level < 1.0 EU/mg as determined by the LAL method Purity ≥95% monomer by analytical SEC ⋅ >95% by SDS Page Formulation This monoclonal antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. Product Preparation Functional grade preclinical antibodies are manufactured in an animal free facility using in vitro cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. Storage and Handling Functional grade preclinical antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at ≤ -70°C. Avoid Repeated Freeze Thaw Cycles. Country of Origin USA Shipping Next Day 2-8°C RRIDAB_2737552 Additional Applications Reported In Literature ? B LAG-3 antibody (clone C9B7W) blocks the function of murine LAG-3 in vivo. Each investigator should determine their own optimal working dilution for specific applications. See directions on lot specific datasheets, as information may periodically change. DescriptionDescriptionSpecificity LAG-3 antibody (clone C9B7W) recognizes and specifically binds to an epitope in the D2 domain of CD223. Background LAG-3 is a 70-kD, type-I transmembrane glycoprotein within the Ig superfamily with four extracellular Ig-like domains (D1 to D4) and is structurally homologous to CD4. LAG-3 is a cell surface molecule with various biologic effects on T cell function. It has been reported to be involved in Treg suppressive function. It negatively regulates cellular proliferation, activation, and homeostasis of T cells, in a similar manner to CTLA-4 and PD-1. Human LAG-3 is approximately 70% homologous with murine LAG3, and it binds MHC class II molecules with higher affinity than CD4. As an immune checkpoint receptor, LAG-3 is the target of various drug development programs seeking to expand treatments for cancer and autoimmune disorders. In its soluble form, LAG-3 is being developed as a cancer drug. As an antagonist, LAG-3 antibody can activate T effector cells via the downregulation of the LAG-3 inhibiting signal into pre-activated LAG-3+ cells. In addition, it can inhibit antigen-specific Treg suppressive activity. As an agonist antibody, it can be used to diminish an autoimmune response and is currently being investigated for the treatment of plaque psoriasis. Antigen Distribution CD223 (LAG-3) is expressed on T regulatory cells, activated T cells and NK cells. NCBI Gene Bank ID UniProt.org Research Area Immunology . Inhibitory Molecules Leinco Antibody AdvisorPowered by AI: AI is experimental and still learning how to provide the best assistance. It may occasionally generate incorrect or incomplete responses. Please do not rely solely on its recommendations when making purchasing decisions or designing experiments. In Vivo Applications of Clone C9B7W in MiceClone C9B7W is a widely used monoclonal antibody that targets mouse LAG-3 (CD223), an inhibitory immune checkpoint receptor expressed on activated T lymphocytes (including both CD4+ and CD8+ T cells), natural killer (NK) cells, and regulatory T (Treg) cells. While C9B7W recognizes an epitope in the D2 domain of LAG-3 and does not block binding of LAG-3 to MHC class II, it is a functional antagonist with several established in vivo applications in murine models. Functional Blockade of LAG-3 Signaling
Mechanistic Insights
Additional Research Applications
Summary Table: Key In Vivo Uses of C9B7W in Mice
ConclusionClone C9B7W is a cornerstone tool in murine immunology, especially for in vivo studies aiming to block LAG-3-mediated immune suppression in cancer immunotherapy, to interrogate Treg function, and to explore LAG-3’s role in immune regulation. Its unique mechanism—functional blockade without preventing MHC class II binding—makes it particularly valuable for mechanistic studies and combination therapies. The antibody C9B7W is most commonly used to detect or functionally block mouse LAG-3 (CD223) in immunology research, particularly in the study of immune regulation and checkpoint blockade. In the literature, it is routinely paired with other antibodies or proteins to characterize immune cell populations or analyze functional pathways. Other commonly used antibodies or proteins with C9B7W include:
Summary Table
These combinations facilitate in-depth investigation into T cell activation, regulatory function, immune checkpoint interactions, and cell phenotyping. Selection depends on the experimental design, such as immunophenotyping (flow cytometry), functional blockade (in vitro/in vivo), or protein quantification (ELISA). If you are seeking a specific panel for flow cytometry or in vivo blockade studies, anti-CD3, anti-CD28, anti-CD4, anti-CD8, anti-PD-1, and MHC-II tetramers represent the most widely reported partners for C9B7W in the literature. Key findings from citations referencing clone C9B7W in scientific literature include:
Overall, C9B7W is a valuable tool for studying LAG-3 function and its role in immune regulation, particularly in the context of T cell activation and inhibition. Dosing regimens for clone C9B7W (anti-mouse LAG-3/CD223) vary significantly depending on the specific mouse model, the experimental context, and the therapeutic goals of the study. While the antibody is widely used for both in vitro functional assays and in vivo blockade of LAG-3, there is no single universal regimen; researchers tailor the dose, frequency, and route based on their model and endpoint. Key details on dosing variation:
The following table summarizes regimen aspects as reported in product datasheets and literature:
Combination and Scheduling:
Summary: References & CitationsTechnical ProtocolsCertificate of Analysis |
Related Products
Prod No. | Description |
|---|---|
S211 | |
R1364 | |
I-1195 | |
C247 | |
F1175 | |
R1214 | |
S571 |
Formats Available
Prod No. | Description |
|---|---|
C2252 | |
C2253 | |
C2254 | |
C2255 | |
C2256 | |
C2249 | |
C2251 | |
C2250 | |
L306 | |
C2155 | |
L502 | |
C2852 | |
L313 | |
L310 |
 Products are for research use only. Not for use in diagnostic or therapeutic procedures.
Products are for research use only. Not for use in diagnostic or therapeutic procedures.